Bradley Haynes and Stark Michael, Physiology and Developmental Biology
Introduction
Both Wnt signaling and neural crest cells (NCCs) have a well-established role in the development of facial bones and cartilage. Wnt signaling is known to be required for neural crest cell formation; however, Wnt expression originating from the neural crest after their specification by Wnt1 has not been carefully explored. The purpose of our study is to investigate the role of Wnt secretion from NCCs in craniofacial development. Our hypothesis is that loss of Wnt secretion from NCCs will lead to improper craniofacial development and contribute to facial structure abnormalities.
Materials and Methods
To study the effects of Wnt secretion from NCCs in orofacial development, we used a Wnt1-Cre mediated deletion of the gene porcn. Porcn is a gene that codes for a crucial O-acyltransferase that modifies Wnt proteins through the addition of the molecule palmitoleic acid. Without this modification Wnt proteins are incapable of being secreted into the extracellular matrix to participate in cell signaling. Creating a porcn knock-out therefore inhibits Wnt signaling.
Using transgenic mice ordered from Jackson labs we bread a population of mice in which the porcn gene was knocked-out by using a cre-lox system. This was done by crossing a male homozygous for Wnt1-cre recombinase with a female homozygous for porcn with surrounding loxp sites.
Upon retrieval of the embryos we genotyped them through PCR to identify which of our embryos were porcn mutants (data not included). This allowed us to then compare the craniofacial development of these mutant embryos to those in which the porcn gene was present.
To view and compare the developmental malformations of our mutants we prepared whole mounts of our embryos and viewed them using whole mount microscopy. In addition to using whole mount imaging and comparison, we used skeletal staining to allow us to view cranial structures through the ectodermal tissue and compare abnormalities. Furthermore, we plan to section craniofacial tissue in both mutant and non-mutant mice to compare palate formation. Finally, immunohistochemistry will be used to view NCCs in the tissue.
Results
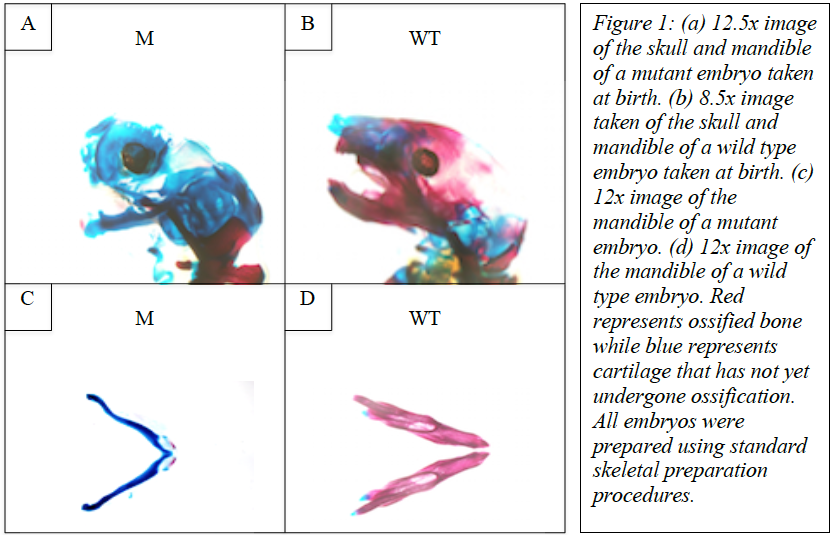
Upon comparing wild type and mutant embryos, we found that ossification was severely lacking in the bones of the skull and other craniofacial skeletal components such as the mandible. This is demonstrated in figure 1. These images were taken of both wild type and mutant mice following skeletal staining.
In addition to bones of the skull and mandible lacking ossification in mutant embryos, the bones of the ear showed significant differences when mutant and wild type embryos were compared. In normal development, the malleus seperates from the mandible; However, embryos that lacked wnt secretion from NCCs and the dorsal neural tube showed that the malleus and mandible remain fused together after birth. This is shown in figure 2.
Conclusions
In conclusion, our research demonstrates that wnt secretion from NCCs and the dorsal neural tube appears to play a critical role in the ossification of the bones of the skull and other craniofacial skeletal components such as the mandible. Furthermore, wnt signaling originating form neural crest cells acts to allow separation of the mandible and malleus, results in the normal anatomy seen in wild type mice.