Benjamin Bitner and Jeffery Tessem; Nutrition, Dietetics, Food Science
Introduction
Increasing a patient’s functional β-cell mass may provide a cure for both types of diabetes. Previous studies have shown that overexpression of the homeodomain transcription factor Nkx6.1 stimulates β-cell proliferation, increases glucose stimulated insulin secretion (GSIS) and decreases apoptosis1. Functional β-cell mass is defined by the number of β-cells, which is dependent on proliferation and apoptosis rates, multiplied by the ability to secrete insulin. Increasing β-cell proliferation or insulin secretion while decreasing apoptosis could be used as a treatment to produce β-cells for islet transplantation, a potential cure for diabetes.
Our research group previously demonstrated that the expression of c-Fos is up-regulated early by Nkx6.1. This indicated that c-Fos is critical for the positive modulations observed with Nkx6.1 in the β-cell. Our research has shown that c-Fos plays an essential role in inducing β-cell proliferation. We hypothesized that c-Fos also enhances GSIS from the β-cell. The prohormone VGF is critical for insulin secretion2, necessary for Nkx6.1 mediated GSIS, and sufficient to enhance insulin secretion from β-cells (Fig 1B). Our lab previously completed microarray analysis of primary rat β-cells 24 and 48 hours after adenoviral transduction. Network analysis of this data indicated that c-Fos is closely linked to VGF expression (Fig 1C). Our group has shown that c-Fos increases VGF expression (Fig 1D). Based on these data, we hypothesized that c-Fos is critical for Nkx6.1 mediated upregulation of VGF and the ensuing increase in GSIS. We tested our hypothesis by determining if c- Fos is sufficient to enhance GSIS and by determining if c-Fos is necessary for Nkx6.1 enhanced GSIS.
Results
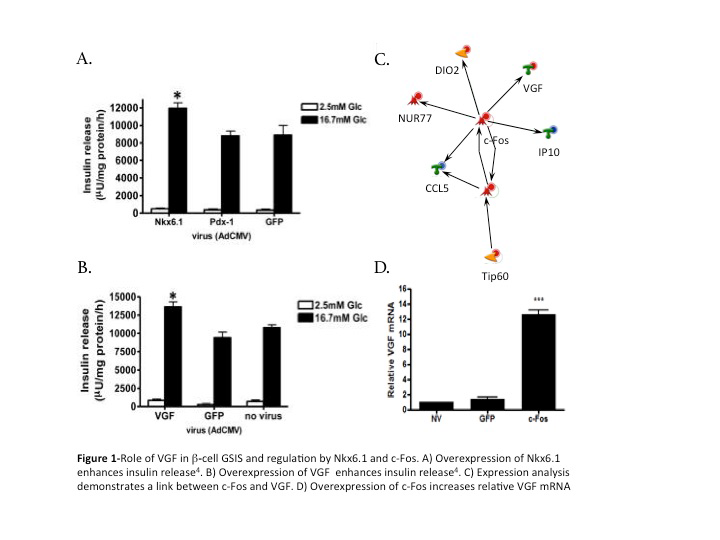
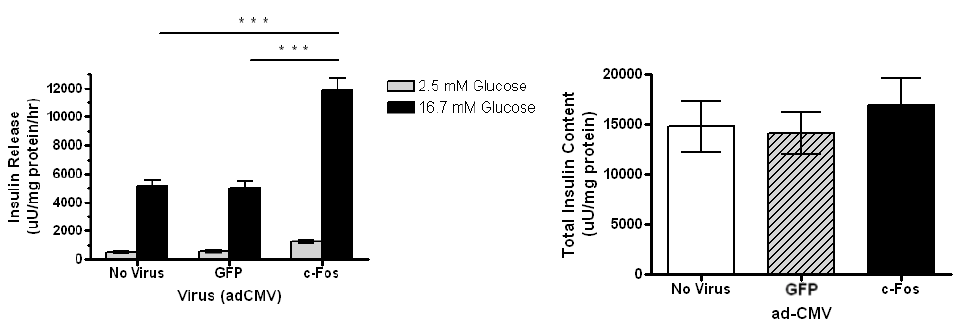
Overexpression of c-Fos enhances glucose-stimulated insulin secretion in beta cells
Insulin secretion in the beta cell is enhanced three fold when transduced with c-Fos compared to no virus and GFP. INS-1 832-13 cells were treated with recombinant adenoviruses (AdCMV) expressing GFP, Nkx6.1, or c-Fos as indicated and analyzed 48 hours post-viral treatment. Data represent the mean ± SEM for five independent experiments. Insulin secretion in adCMV-c-Fos-treated cells was significantly enhanced (P = 0.0292), and total insulin content remained constant.
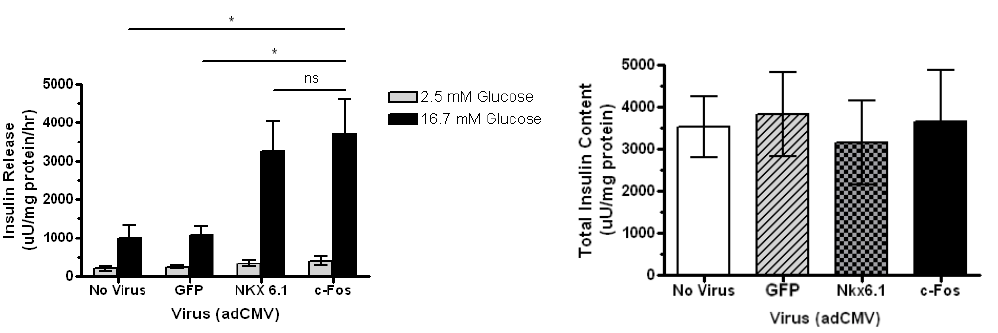
Suppression of c-Fos expression impairs glucose stimulated insulin secretion in beta cells
With the lack of c-Fos, insulin release and total insulin content is decreased. This suggests that c-Fos is required for GSIS. INS-1 832-13 cells were treated with Ad-sic-Fos or Ad-siGFP viruses followed by overexpression of Ad- CMV-Nkx6.1 and analyzed 48 hours post-viral treatment. Glucose-stimulated insulin secretion and total insulin content were measured by static incubation in media containing 2.5 mM glucose and 16.7 mM glucose for 1 h each. Data represent the mean ± SEM for three independent experiments. Insulin secretion in Ad-sic-Fostreated cells was significantly reduced in both basal and high glucose (p = 0.0263 and p= .0233, respectively), and total insulin content was significantly reduced (P < 0.001).
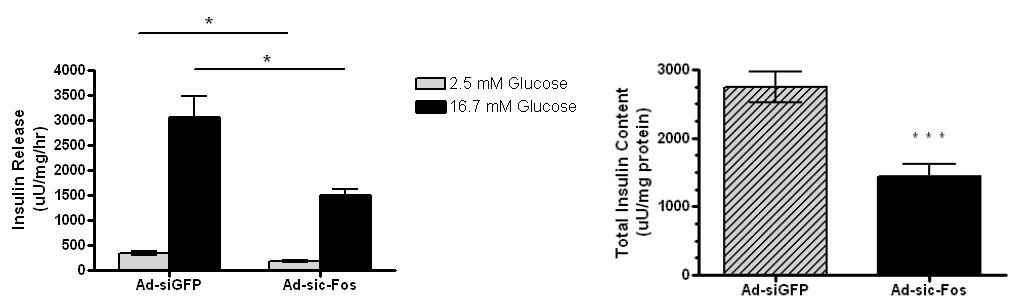
Overexpression of c-Fos enhances GDID in primary rat islets
Transduction of islets with c-Fos enhances GSIS while maintaining the total insulin content. Rat islets were treated with AdCMV expressing GFP, Nkx6.1, or c-Fos as indicated and analyzed 48 h post-viral treatment. Data represent the mean ± S.E.M of 5 independent experiments. Insulin secretion in adCMV-c-Fos-treated cells was significantly enhanced (P < 0.0001) and total insulin content remained constant.
Methodology
Cell Culture. INS-1 derived 832/13 rat insulinoma cells were maintained in complete RPMI 1640 medium with Lglutamine and 11.2 mM glucose supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin, 10 mM HEPES, 10% fetal bovine serum, and INS-1 supplement. HEK 293 cells, used for lentivirus production, were maintained in DMEM medium with 50 U/ml penicillin, 50 μg/ml streptomycin, and 10% fetal bovine serum.
Glucose stimulated insulin secretion. Cells were grown to confluence, washed with PBS and preincubated in secretion assay buffer (SAB) for 1.5 hours (114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4 1.16 mM MgSO4, 20 mM HEPES, 2.5 mM CaCl2, 0.2% BSA, pH 7.2) containing 2.5 mM glucose. Glucose stimulated insulin secretion (GSIS) was performed by incubating quadruplicate replicate wells of cells in SAB containing 2.5 mM glucose for 1 hour, followed by 1 hour in SAB with 16.7 mM glucose, followed by collection of the respective buffers. For total insulin content, cells were lysed in RIPA buffer with protease inhibitors (Life Technologies). Secreted insulin and total insulin was measured in SAB using a rat insulin RIA kit (MP Biomedicals).
Lentivirus production and gene knock down. Rat shRNA constructs against c-Fos (sh-c-Fos: 5’- AGACCGAGATTGCCAATCTACTGAAAGAG-3’) and a non-effective 29-mer shRNA cassette (termed shCONTROL) were cloned into the pGFP-C-shLenti plasmid (OriGene). Lentivirus was produced by transfecting HEK 293T cells with the respective pGFP-C-shLenti plasmid and packaging plasmids using the Origene Lentiviral Packaging Kit and MegaTran. 832/13 cells were transduced with recombinant lentivirus at a MOI of 3 viral particles/cell, and cultured for 48 hours. Following infection transduced cells were selected through culture with puromycin for two weeks (1 μg/ml), after which cells were transferred to normal culture media for experimental conditions.
Statistical methods. For analysis between two groups, Student’s t-test was used, and differences were considered significant when p<0.05. One-way analysis of variance (ANOVA) was used in experiments that had three or more groups. Differences within ANOVA were determined using Tukey’s post hoc tests, where differences were considered significant when p<0.05. All data are reported as means±S.E.M.
Discussion and Conclusion
Diabetes results from the eventual destruction of β-cell mass, which causes decreased insulin secretion. This is an issue because of the extremely low level of endogenous β-cell proliferation observed in rodent and human islets under normal conditions. While β-cell replication appears to be highly regulated and controlled, human studies have shown clear replication in β-cells from obese individuals and pregnant women3, 4 These data demonstrate that the molecular pathways permitting β-cell replication are intact, and suggest that manipulation of these pathways may be sufficient for ex vivo expansion of β-cell mass for transplantation therapy or in vivo expansion of residual β-cells. Therefore, defining the molecular pathways that enhance functional β-cell mass is essential.
Collective data show that c-Fos is necessary for Nkx6.1 mediated β-cell proliferation enhancement of GSIS and protection against apoptosis. My data demonstrates that c-Fos is sufficient to enhance GSIS. Furthermore, our data demonstrate that overexpression of c-Fos is sufficient to induce expression of VGF (which is critical for increased insulin secretion). Finally, my data show that c-Fos is necessary for Nkx6.1 mediated β-cell enhancement of GSIS. These data place c-Fos as a critical link between the β-cell transcription factor Nkx6.1 and its ultimate ability of increasing functional β-cell mass.
While no data have yet directly linked c-Fos to β-cell proliferation or insulin secretion, transcriptional targets of c-Fos are linked to both of these phenotypes. In conclusion, this study reports for the first time that c-Fos is necessary and sufficient to enhance glucose stimulated insulin secretion, a critical component of functional β- cell mass. Additionally, this study further defines c-Fos as a component that may be manipulated to enhance GSIS for islet transplantation to treat patients with diabetes.
References
- Schisler, J.C., et al.(2008). Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol 28: 3465-76.
- Stephens, S.B., et al. (2012). A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet beta-cell survival and function. Cell Metab 16: 33-43.
- Butler, A.E., Cao-Minh, L., Galasso, R., Rizza, R.A., Corradin, A., Cobelli, C. and Butler, P.C. (2010). Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 53, 2167-76.
- Saisho, Y. et al. (2010). Relationship between fractional pancreatic beta cell area and fasting plasma glucose concentration in monkeys. Diabetologia 53, 111-4.