David Lee and Dr. Dixon Woodbury, Physiology and Developmental Biology
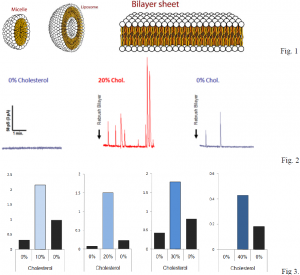
A bilayer is a two-layered membrane made of lipids that form around cells. These membranes are formed by hydrophobic tails (water fearing) and hydrophilic heads (water loving) that align such that the tails are isolated from water, while the heads are exposed to water to form micelles, liposomes, or sheets (Fig. 1). They are used to regulate what can enter and exit a cell. A fusion event occurs when a liposome fuses with a sheet. In our experiments, we are able to see the effects on fusion rate by altering the cholesterol content of the sheets.
Our hypothesis was that by increasing cholesterol content, we would see an increase in fusion rate. This is precisely what we observed. After forming an artificial membrane that would have 0% cholesterol and would not observe a significant amount of fusions, we added cholesterol in specific amounts and would observe an increase in fusion rates (Fig. 2). For further verification of our data, we would increase the cholesterol content, then reduce it, then increase it again and would see a proportionate increase or decrease in rates of fusion. We compiled our data and represented rates in terms of fusions per minute with respect to cholesterol content in the bilayers (Fig. 3).
Our hard work resulted in a poster that we created and presented at the 54th Annual Meeting for the Biophysical Society that was held in San Francisco, California. We were able to meet thousands of biophysicists from around the world and not only share our data with them, but collaborate with them while we studied what they were working on. We were also able to see the cutting edge technology that is being used in research labs, and get new ideas for future projects.
Although our experiments were simple in design, results were hard to procure because of several difficulties. Each experiment would take several hours, and one mistake during the experiment could render the entire experiment void. We would “paint” our bilayers on plastic cups with microscopic holes. These plastic cups would get chips and cracks which resulted in faulty data. Also, we experienced technical difficulties with the computer which would freeze and cause us to discard that particular set of data. Contamination or expiration of the solutions used in our experiments also resulted in long delays because we would have to identify which solutions were contaminated and then replace them.
A deeper knowledge about liposomes and bilayers would be beneficial because liposomes are an ideal drug delivery system. Drugs can be placed in the space that is formed from the hydrophobic tails of the liposomes and can be released into target specific areas. This would be beneficial for cancer treatments, depression, and various other diseases and disorders.
One of the ideas that we gained from attending the Biophysical Society conference was another method to form bilayers. Instead of forming the bilayers on the holes of the plastic cups, we are now forming them on the tip of a glass pipette. We are still perfecting this technique, but we have gathered enough data for another poster that we will be presenting at the next Biophysical Society conference that will be held in Baltimore, Maryland next year.
We hope that the culmination of all our data will result in a research paper that will be published in a mainstream scientific journal.