Jakob Gamboa and Dr. Jonathan Wisco, Physiology and Developmental Biology
Ligaments are specialized connective tissues that stabilize the different synovial joints found in the body. Ligaments consist of fibroblast cells surrounded by a framework of dense fibrous bands of collagen, which anchor to bones to provide support and elasticity. Their varying biomechanical functions in different locations of the body require diversity in matrix composition, shape, density, and arrangement to adapt to the complex functions they perform. These characteristics may even vary within different regions of the same ligament. Damage to ligaments compromises integrity and movement of the joint and can lead to serious complications.
One such ligament prone to injury is the ulnar collateral ligament (UCL) of the elbow. This ligament stabilizes the medial side of the elbow by maintaining the structural relationship between the humerus and the ulna bones of the arm. Athletes who perform overhead throwing activities, such as professional baseball players, are prone to injury of the UCL. Upon dislocation or repetitive stress to the joint, the ligament may experience increased laxity (looseness) or tears. In severe cases of trauma, reconstructive surgery is necessary. Performed first in 1974, the surgery was performed on Los Angeles Dodgers’ pitcher, Tommy John, by replacing the ligament with a tendon from somewhere on the patient’s body1. Since then, the surgery has been colloquially termed Tommy John’s Surgery, and many different procedures have been developed helping many athletes return to former level of performance.
Various procedures have been used to treat the injured UCL. Yet studies report as low as 70% of athletes returning to previous level of play2. While much of the gross anatomy of the ligament is known, little research has been done on the microarchitecture of the arrangement of the fibers to assess injury or to determine which surgical technique may be most effective at repair. Through reconstructing a model of the fibers of the ligament, we provide useful data for that informs of fiber structure, interaction, and injury for clinical applications.
The size and complexity of the ulnar collateral ligament makes gross observation of the fibers nearly impossible. For this reason, no data-driven reconstruction has previously been created. While slicing the ligament into multiple sections to be segmented would create a virtual model, we are unable to demarcate the cross sections of the fibers. However, using a blue stain developed in Dr. Jonathan Wisco’s research lab, we are able to distinguish the fibers along the length of the ligament and virtually traced the structures using advanced technology.
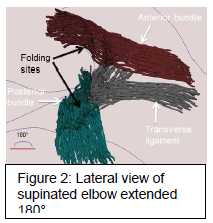
The anterior and posterior bundle of the left UCL ligaments of a 58 year-old cadaveric specimen were dissected and stained to reveal the fibers. Through magnifying lenses and various light sources the individual fibers were distinguished. These fibers were traced into spatial reference points using the Microscribe 3D Digitizer create virtual dotted lines. Fibers were traced in multiple locations along the length, and adjoining lines were connected to ensure a complete model. This process was repeated at with the elbow joint oriented at 90 degree, 135 degree, and 180 degree positions. These reference lines were reconstructed into three-dimensional renderings with the Autodesk Maya animation program.
The fibers continue along a complex arrangement that vary within the ligament, and by position of the joint. Through the amplified model we observe that the fibers display an orderly parallel arrangement at origins and insertions with a greater number of individual fibers that collect into larger fiber groups in the length of the ligament. The filaments interweave in the areas of highest mobility, and deviate from parallel origin, and there is a variation in fiber diameter, determined by location on each bundle. The smallest fibers at attachments average .08 to .1 mm, while larger cords at edges of bundles and areas of greatest stress average .2 mm. Upon movement to different position, bundles have areas designated for folding displacement, which correspond to less ordered areas of the ligament, and the greatest extension and stress of bundles is at 90° angle.
Variation in arrangement significantly affects the strength and mobility of the ligament. With a structure that is more interconnected and interwoven, the ligament would experience the greatest ability to undergo movements as it is reinforced from multiple angles. Increased thickness would also increase the amount of strain that the structure could withstand. Therefore, the model informs of the section of least strength near the sites of attachment. This correlates to common sites of UCL tear. Tracing the virtual lines at different positions also illustrates more clearly the specific locations within the ligament where the folding displacement occurs upon flexion. With greatest valgus strain at the 90 degree position as opposed to extension, the injury will most likely occur as the athlete is accelerating through the movement, as opposed to hyperextending.
 The detailed model provides data for the study of the microarchitecture of the ligament, and reveals variation in size and shape within a complex arrangement of fibers. The arrangement of fibers has implications to not only the function of the ligament, but the areas susceptible to injury. This information is clinically relevant as the non-parallel fiber arrangements advise specific sites for more effective suturing in direct repair reconstructive surgery, and inform to structures that most resemble the structure and function of a healthy UCL. Understanding the nature of the injury can also lead to preventative training and adjustments.
The detailed model provides data for the study of the microarchitecture of the ligament, and reveals variation in size and shape within a complex arrangement of fibers. The arrangement of fibers has implications to not only the function of the ligament, but the areas susceptible to injury. This information is clinically relevant as the non-parallel fiber arrangements advise specific sites for more effective suturing in direct repair reconstructive surgery, and inform to structures that most resemble the structure and function of a healthy UCL. Understanding the nature of the injury can also lead to preventative training and adjustments.
1“Tommy John Surgery (UCL Reconstruction) and Recovery.” WebMD. WebMD, n.d. Web. 27 Oct. 2013.
2Vitale. “The Outcome of Elbow Ulnar Collateral Ligament Reconstruction in Overhead Athletes: A Systematic Review.” NCBI. U.S. National Library of Medicine, n.d. Web. 31 Oct. 2013.
