Brett Gardiner and Dr. Jon Wisco, Department of Physiology and Developmental Biology
Introduction
Neuroanatomy lab specimens are limited to cadaver availability and inconsistently demonstrate variations confronted in pathology. Using rapid prototyping (RP) technology to create 3D models from segmented MRI data offers distinct benefits to medical education. An efficient and replicable procedure for customizing these models can be developed to provide unique visual experiences for students and offer additional perspectives of brain models from real data.
Methods
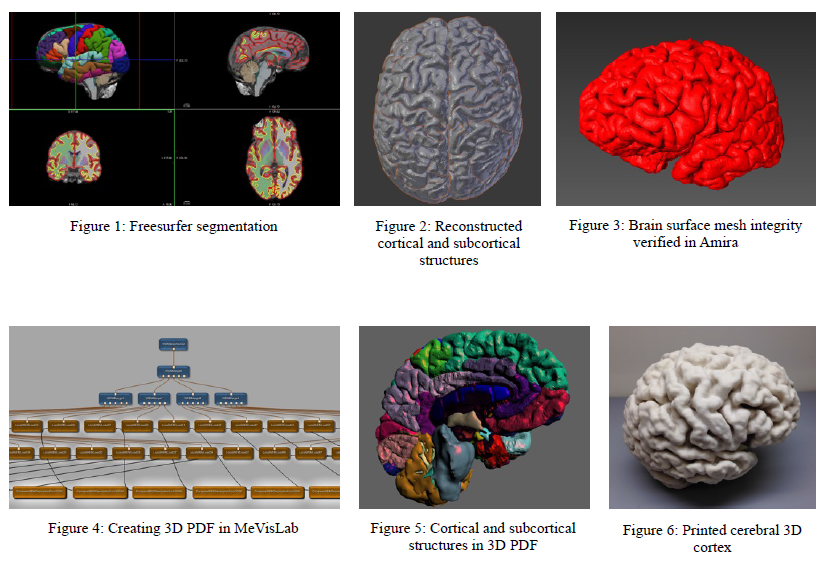
Our methods consist of (1) image segmentation, (2) post-processing lobular isolation and slice manipulation, (3) mesh optimization and (4) rapid prototyping. We segmented cortical and subcortical structures with Freesurfer (Figure 1). From our demarcation, we generated our surface descriptions as a triangulated stereolithography format (STL) mesh by using Areal interpolation scripts (Neuroimage, 2012;61(4):1428-43) to convert from segmented annotations. Reconstruction modules employed by computer graphics (CG) software Blender and 3D segmentation software Amira (Figure 3) were used to improve mesh quality. We corrected inverted normals, intersecting triangles, bad edges and contours, holes, and noise shells.
Advanced CG software modules that solidify mesh surfaces are incapable of maintaining triangle integrity while completing surfaces for RP. Many overlapping triangles persisted. As a companion guide to our RP models, we used MeVisLab (Figures 4) to generate a 3D surface model for embedding into a 3D portable document format (PDF) as shown in Figures 5.
After improving the mesh quality of our labeled cortical into various visual elements. Techniques to improve integration of cortical and subcortical structures into one RP-ready construction and methods to maintain mesh quality while completing the cortical surfaces into a non-manifold printable format are still being explored. Once our desired volumes were sufficiently modeled digitally, we converted our mesh into an RP-ready format and prepared the data for a 3D printer.
Results
Meshes with persisting overlapping faces rendered printing errors as missing layers (Figure 3). Our finalized brain models demonstrate neuroanatomy otherwise unavailable to the classroom. We are able to print brain structures (Figure 6) as educational models. The companion 3D PDF contains a model tree with view options to demonstrate delineated brain structures (Figure 5). This further facilitates our goal for platform-independent visualization tools for educational accessibility.
The models respect the morphological integrity portrayed by interpolated and normalized data. Printing costs do grow exponentially as the printed volume increases; however, by extracting segmented cortical structures of interest, large portions of costs can be eliminated, and models specific to classroom objectives can be printed to specification. Further economic efficiencies can be explored including downscaling and hallowing models to reduce print material.
Conclusions and Discussion
By manipulating a surface mesh rendered from MRI segmentation, anatomical varieties can be modeled as handheld specimens. These demonstrations are advantageous for education over artistic interpretation because they are representations of real data. Through the pipeline techniques we propose, we can articulate morphological neuropathologies and provide unique and modular perspectives for neuroanatomy education. Methods to bridge the gap between real medical data and CAD are being explored in order to improve RP measures and surface preparation for 3D printing.