John Hancock and Dr. Scott Weber, Department of Microbiology and Molecular Biology
Introduction
CD4+ helper T-cells play a vital role in the body’s immune response. When infectious agents attack the body, phagocytes engulf these invaders and present a peptide segment of the pathogen on a receptor (called MHCII). These receptors are located on the surface of the cell and the displayed peptide is termed an epitope. CD4+ T-cells with T-cell receptors (TCRs) specific for the displayed peptide bind to the MHCII complex. It is this binding that releases chemical signals to initiate an immune response. A disadvantage of TCRs is that their wild-type affinity for MHC is low and that they are membrane bound; not normally being secreted from the T-cell membrane. TCRs that are soluble, stable independent of the cell membrane, and have high affinity for a certain epitope can be localized at the area of infection for a longer period of time. The overall purpose of this project is to engineer a stable, soluble, high affinity T-cell receptor for the epitope from a specific bacterium (Listeria monocytogenes). The hypothesis is that developing these high affinity, soluble receptors will improve TCR targeting to a specific epitope and enable enhancement of the immune system. This could allow for the development of future therapeutics targeting the epitopes of cancers, autoimmune diseases, and viral infections.
Methodology and Results
Dr. Scott Weber had previously developed two T-cell receptors specific to Listeria monocytogenes called LLO56 and LLO118. This project focused on LLO56. These two TCRs are specific for the same epitope and differ by only 15 amino acids but have dramatically different primary and secondary responses to infection. The main approach to this project was to utilize the process of yeast surface display. The DNA for LLO56 was ligated into a yeast display vector called pCT302. The alpha and beta variable chains of the LLO56 TCR protein were then grown as a single chain fused to a protein called Aga-2 on the surface of the yeast cell. The development of a stable, high affinity TCR was done through an evolutionary process of mutagenesis, expression, and selection. This process will be repeated twice: once for the selection of the most stable proteins and again for the most high-affinity.
After LLO56 had been ligated into pCT302, error-prone PCR (polymerase chain reaction) was used to insert different mutations into the gene. PCR is a process that amplifies a specific segment of DNA. This process was made “error-prone” by supplying differing amounts of the four DNA nucleotides. Primers were developed to target LLO56 and a large mutant TCR library was developed. This library was then introduced into yeast through homologous recombination and the most stable, high-affinity mutant proteins could then be selected.
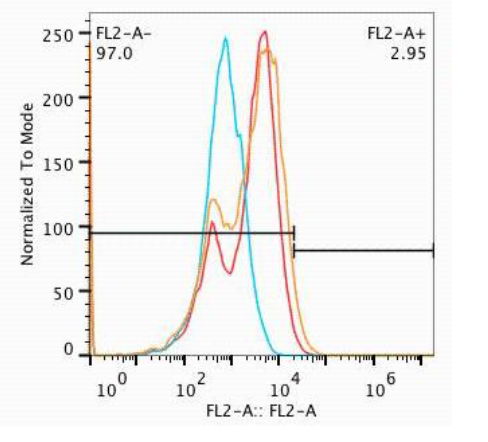
The most stable proteins were selected for with fluorescently labeled, biotinylated antibodies that targeted the variable portions of the TCR. The sample was passed through a column of anti-biotin beads which sorted the mutant proteins according to their stability. Two generations of selection were accomplished and the results were analyzed by flow cytometry1. The most stable TCR mutants were sequenced to identify mutations and then will move on to be screened for high-affinity.
Discussion and Conclusions
The selected stability TCR proteins will soon be undergoing high-affinity selection. A mutant-TCR library will be developed through site-directed mutagenesis of the CDR3 region (the part of the variable portion of the TCR that determines specificity and interacts with the MHCII) of the LLO56 gene. The same process of yeast surface display will continue as above and the highest affinity TCRs will be selected for with a MHCII tetramer. The most stable, high affinity mutant TCRs can then be isolated, sequenced, and tested against Listeria monocytogenes. The results could lead to a potential therapeutic that should more effectively target and slow the effects of L. monocytogenes infected cells.
The results with LLO56 can also be compared to research being done with LLO118. As stated previously, the two TCRs have very different primary and secondary responses to infection. One very important future direction that this research can take includes further discoveries into how immune memory operates.
1The second generation LLO56 TCR protein mutants (orange) expressed on the surface of yeast at higher levels than the first generation (red) and the wild type (blue). The selection was done with antibodies for the variable beta TCR chain. The shift seen in the second generation indicates an increase in stability. Selection for the variable alpha TCR chain will soon follow.