Jacob B. Hatch and Bradford Berges, Ph.D., Microbiology and Molecular Biology
Introduction
About 20% of humans are carriers of Staphylococcus aureus (SA). There were an estimated 11,000 deaths in the United States in 2005 attributed to SA, with the majority caused by MRSA (Methicillin Resistant Staphylococcus Aureus) isolates [1]. Many MRSA isolates have developed resistance to all but one antibiotic drug: vancomycin. However, other bacteria have developed resistance to vancomycin, suggesting that in time MRSA will likewise become non-responsive to this last available drug and MRSA infections will be untreatable. This project looks to find an alternate method of MRSA treatment. Bacteriophage (phage) are viruses that infect bacterial cells in order to self-replicate and produce new progeny, thus lysing and killing bacterial cells. The idea of using phage as a potential therapeutic tool has been around for as long as phage have been known to exist [2,3]. Although bacteria can evolve to escape from phage killing, the use of a biological agent such as phage allows for evolution to also work in favor of the phage to re-acquire the ability to kill target cells. Thus, it is thought that phage therapy could be superior to antibiotic therapy in terms of the ability of the treatment to evolve in response to the development of resistance by the target bacterium. Our hypothesis is that bacteriophage can serve as an alternate method to control pathogenic bacterial infections, and that this will be especially useful for bacterial species that are highly resistant to antibiotic therapy.
Methodology
Our research has consisted of encountering and isolating the phage, performing electron microscopy of the virions, and determining each isolate’s killing effectiveness, with the goal of creating a way to control MRSA. To select for staphylococcus aureus bacteria, each sample was suspended in LB broth, vortexed, plated on mannitol salt agar and incubated for 48 hours. Methicillin resistance was determined by plating strains on mannitol salt agar with 2 micrograms per milliliter oxacillin, and then incubated for 48 hours. Bacteria growing on these plates were considered S aureus and MRSA, respectively. Having successfully isolated these bacterial strains, we then looked to isolate bacteriophage with the ability to kill them. To isolate lysogenic phage, log-phase cultures were grown for 30 minutes at 37º C and then exposed to mitomycin C, a DNA-crosslinking agent. Cells were pelleted and the supernatant filtered, then plated against its target bacteria, with plaques indicating successful killing. To isolate non-cell associated phage, enrichments were created and purified, then plated against target bacteria.
After isolating each bacteriophage, at least three rounds of plaque purification were completed to ensure that each strain was indeed only one bacteriophage, and not multiple. Having purified these strains, high titer lysates were produced by performing serial dilutions, plating, and counting plaque-forming units. High titer lysates are necessary for DNA extraction and to perform transmission electron microscopy. Preparation of samples for electron microscopy was performed by pipetting 50 μL of high titer lysate and phosphotungstic acid onto a small piece of parafilm in separate drops. Then, floating a copper mesh grid on each drop using microforceps for 1.5 minutes before wicking the moisture away. Microscopy was performed on a Tecnai T-12 transmission electron microscope.
Results
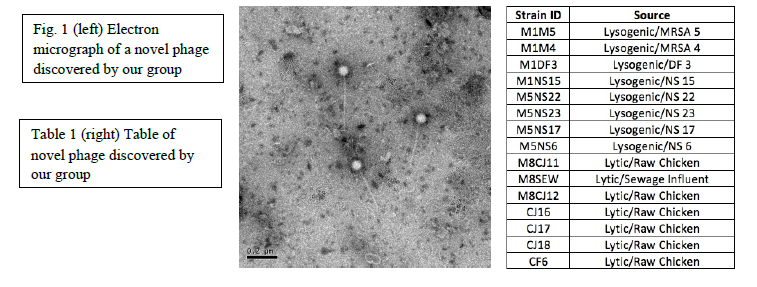
During the last year we have discovered 15 potentially novel bacteriophage that have shown the ability to kill MRSA (examples shown in Fig. and Table 1, below). These phage were isolated from a number of environmental sources including athletic facilities, dog fur, raw chicken, hospitals, sewage water, and the human nose. Transmission electron microscopy (TEM) has been performed on 8 of these phage, and we are currently determining the ability of each bacteriophage to kill various strains of MRSA.
Discussion
TEM images produced have aided in our viewing the morphology of the strains isolated. Thus far, all seem to belong to the siphoviridae family based on capsid and noncontractile tail sizes. Preliminary tests to determine the killing power of each strain of bacteriophage have proven inconsistent, and further testing will be necessary to determine the killing ability of each phage with certainty. Completion of this next step will allow the creation of a cocktail of phage strains with the ability to kill various MRSA strains, with numerous applications within the medical field.
Conclusion
Fifteen potentially novel bacteriophage strains were isolated within the last year, with TEM imaging completed for eight. We have not yet proven whether bacteriophage can serve as an alternate method to control pathogenic bacterial infections, however, early results have confirmed this possibility.
Scholarly Sources
- Klein, E., D.L. Smith, and R. Laxminarayan, Hospitalizations and deaths caused by methicillinresistant Staphylococcus aureus, United States, 1999-2005. Emerg Infect Dis, 2007. 13: p. 1840-6.
- Burrowes, B., et al., Bacteriophage therapy: potential uses in the control of antibiotic-resistant pathogens. Expert Rev Anti Infect Ther., 2011. 9: p. 775-85.
- Sulakvelidze, A., Z. Alavidze, and J.G.J. Morris, Bacteriophage therapy. Antimicrobe Agents Chemother, 2001. 45: p. 649-59.
