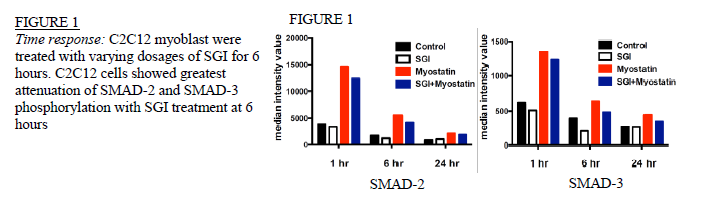
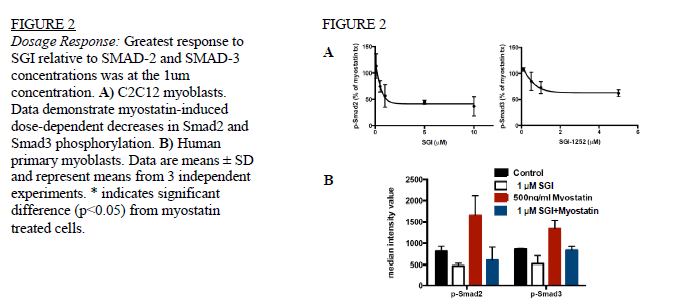
Ryan Matekel and Robert Hyldahl, Exercise Sciences
Introduction
Muscle wasting is a symptom of cancer, AIDS, renal failure, heart failure, aging, prolonged bed rest, and has been seen in rodent models for burn, and kidney disease (Han, 2013). Muscle wasting negatively impacts quality of life by decreasing functional independence and increasing morbidity and mortality (Anker, 1997). Myostatin is a protein that negatively regulates muscle mass and is primarily expressed within skeletal muscle (Lee & McPherron, 2001). It exerts its effect by binding to a receptor and initiating a signaling cascade that involves phosphorylation of two kinase proteins called SMAD-2 and SMAD-3 (Sartori et al., 2009). Genetic studies have shown that inhibition of myostatin signaling may be an effective way to attenuate muscle wasting (Heineke et al., 2010). However, a pharmacologic agent capable of inhibiting myostatin has not yet been identified. In this study, we performed in vitro analyses to test the effectiveness of a novel small molecule (SGI) in inhibiting myostatin signaling in a cultured muscle cell model. Specifically, we determined how SGI dose and treatment time affected phosphorylation of SMAD-2 and SMAD.
Methodology
Cell Growth
Using 10 cm petri dishes we cultured C2C12 mouse myoblasts in a medium that consisted of a base of DMEM with 10% fetal bovine serum. Cells were allowed to grow to a confluency of 70-80% before passaging into new dishes. Prior to treatment, cells were passaged into 6-well plates and allowed to grow to 80% confluency. Human myoblasts were cultured in the same manner but with 20% fetal bovine serum.
Treatment
Time Response: Cells in 6-well plates were divided into 4 treatment groups and analyzed over time periods of 1, 3, 6, 12, and 24 hours. Treatment groups consisted of: 1) cells treated with SGI, 2) SGI control (treated with equal concentrations of solubilization agent), 3) cells treated with myostatin and 4) cells treated with both SGI and myostatin. Dose Response: C2C12 and human myoblasts were treated in the same 4 groups as the time response. The 4 groups were treated with 100nm, 500nm, 1μm, 5μm, 10μm, 30μm and 100μm concentrations of SGI and harvested after 6 hours.
Analysis
Collected proteins from both trails were analyzed using the same methodology. After harvesting the proteins using a lysis buffer, we determined the concentrations of protein in each well using a standard BCA assay. We then used a magnetic bead immunoassay to measure concentration of total and phosphorylated proteins involved in the myostatin signaling pathway.
Results
Discussion
This experiment was a preliminary test to determine whether SGI would be a viable candidate as a potential therapy for muscle wasting disease. The data show that SGI effectively inhibit the myostatin signaling pathway, suggesting that it may be a viable pharmacological intervention for the treatment of muscle wasting disorders. Further experimentation will be required to uncover the effectiveness of SGI in increasing muscle growth and preventing muscle loss in models of muscle wasting or disease.
Conclusion
If SGI can be proven to increase muscle mass, then the mortality rate of those who suffer from muscle wasting diseases or problems (muscular dystrophy, cancer cachexia, old age, bed rest, etc.) will be lessened as healthy muscle will be able to grow and improve the overall health of the affect person.
Sources
Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet1997;349:1050–3.
Han HQ, et al. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int J Biochem Cell Biol (2013), http://dx.doi.org/10.1016/j.biocel.2013.05.019
Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010;121:419–25.
Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proceedings of the National Academy of Sciences 2001;98:9306–11.
Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, et al. Smad2 and3 transcription factors control muscle mass in adulthood. American Journal of Physiology-Cell Physiology 2009;296:C1248– 57.