Camilo Mejia and Juan Arroyo, PhD, Physiology and Developmental Biology
Introduction
Pregnancy necessitates interactions between the mother and the fetus, and the placenta is the medium through which this is accomplished. Many complications during pregnancy, such as intrauterine growth restriction (IUGR) and preeclampsia (PE) arise from abnormalities in trophoblast cells of the human placenta. Trophoblast cells form the connection between the fetus and the uterine wall. We see placental hyperosmolarity in pregnant patients with diabetes, and there are some reports of aberrant trophoblast functioning during IUGR and PE at term, but more needs to be studied regarding the hyperosmolar stress associated with these pathologies and its role in embryo development. Deviation from normal trophoblast function may lead to impaired oxygen and nutrient exchange and may play a significant role in the development of these diseases which include decreased trophoblast invasion and an increased trophoblastic apoptosis. Hyperosmolarity has been seen in placental tissues from patients with gestational diabetes and PE.
NFAT5 (nuclear factor of activated T cells) is a protein that ensures the normal development of neural and muscle tissue, and in the synthesis of second messengers during pregnancy. It also modulates cellular response to osmotic changes by accumulating inositol and sorbitol inside the cells. Cells that lose intracellular water when subjected to hypertonic stress adapt by accumulating organic osmolytes (like inositol and sorbitol) to re-establish osmotic equilibrium. This may occur in response to a hypertonic environment or stress encountered by the trophoblast cells in the interface between the uterus and placenta. Indirect evidence for such hypertonic stress occurring at the time of trophoblast invasion has been provided, mainly by showing hypertonic stress induced mRNA responses in trophoblast stem cells, which emulated postimplantation differentiation1. NFAT5 has been shown to stimulate production of the sodium-myo-inositol cotransporter (SMIT) and the aldose reductase (AR). A reasonable mechanism by which the placenta accumulates inositol and sorbitol would be through the action of NFAT5 and the downstream effects on SMIT and AR. We investigated the effects of hyperosmolarity during pregnancy by observing trophoblast behavior and the expression of certain proteins involved in hyperosmolar-induced osmolyte accumulation. We chose to work with the Swan 71 (SW71) cell strain, which is derived by telomerasemediated transformation of a 7-week cytotrophoblast isolate, and has a phenotype that resembles extravillous trophoblasts2. Extravillous trophoblasts are in close contact with the uterine wall and may be exposed to the hyperosmolar conditions that could indicate the placental pathologies previously mentioned.
Methodology
SW71 cells were plated at a density of 30,000 cells/well in a 12 well plate and allowed to grow to approximately 70-80 % confluence over 2 days. On day 3, fresh control media was added to the control samples (n=4) and 14.6% NaCl hyperosmolar solution was added to the treated samples (n=4) for 6 hours. The measured osmolarity of the control media was 273 mOS. NaCl was chosen to induce hyperosmolarity in the present study because it is normally present in the interstitial fluid of tissues3 and was thought to be the most likely solute encountered by invading trophoblasts at the uteroplacental interface. To determine the appropriate concentration of treatment multiple experiments using different osmolarity concentrations and different durations of exposure were performed. The hyperosmolar concentrations included 450, 670, and 1230 mOS. The duration of the experiments ranged from 2, 4, 8, and 24 hours. Cells showed visible signs of apoptosis by 8 hours of culture and a concentration of 450 mOS NaCl. After exposure to these conditions, cells were lysed and scraped. Western blots and immunofluorescence were performed against SMIT and AR proteins. All data was subjected to statistical analysis using t-tests for comparison between groups. Significance was set at p ≤ 0.05. Immunofluorescence was performed on cultured SW71 cells treated with control and hyperosmolar media using chamber slides. In summary, slides were frozen in -80°C overnight and then fixed for 20 minutes in -20° C acetone. Slides were blocked with Sniper and incubated overnight with a rabbit polyclonal primary antibody against NAFT5, SMIT, and AR along with a negative control. Slides were incubated with anti-rabbit Dylight fluorescent polymer.
Results
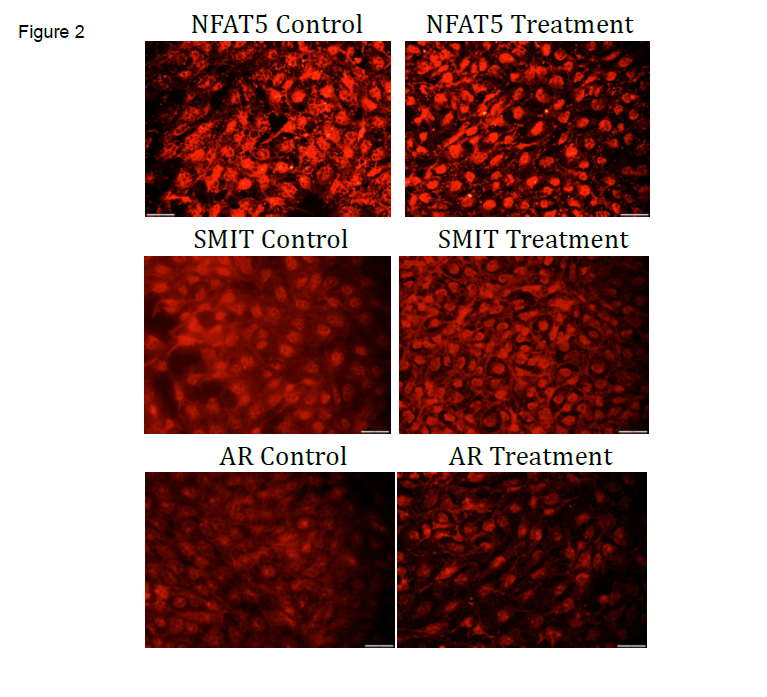
Overall we observed: 1) an increase of AR expression in the SW71 cells treated with hyperosmolar media (see Figure 1) with no significant increase or decrease in SMIT expression, and 2) increased nuclear expression of NFAT5, SMIT, and AR in SW71 cells treated with hyperosmolar media (see Figure 2).
Discussion and Conclusion
The results we observed showed the response of trophoblast cells that come in contact with hyperosmolar conditions. Hyperosmolar conditions can be encountered during diabetes, IUGR, or PE. SW71 cells showed an increase in nuclear expression of the key proteins in the pathway that leads to osmolyte accumulation when exposed to hyperosmolar media. Hence the NFAT5, SMIT, AR is a nuclear pathway that produces inositol and sorbitol, two important osmolytes that help keep the cell homeostatic under hyperosmolar conditions. Understanding this mechanism can help create therapies and prevent complications from the pathologies afore mentioned. These findings have helped our research expand, and we are currently investigating other proteins that could play a role in this important pathway.
1Liu J, Xu W, Sun T, Wang F, Puscheck E, Brigstock D, Wang QT, Davis R, Rappolee DA. Hyperosmolar stress induces global mRNA responses in placental trophoblast stem cells that emulate early post-
2Aplin JD, Forbes SL, Straszewski-Chavez B, Kalionis C, Dunk D, Baczyk K, et al. Trophoblast differentiation: Progenitor cells, fusion and migration – A workshop report. Placenta. 2006; 27: 141-3.
3Fogh-Andersen N, Altura BM, Altura BT, Siggaard-Andersen O. Composition of interstitial fluid. Clinical Chemistry 1995; 41: 1522-5.