Jason Ray and Jeffery Tessem, Department of NDFS
Introduction
Type 1 and Type 2 diabetes are major global health concerns. Both types of diabetes result in loss of functional β-cell mass, which is defined as the β-cell number multiplied by insulin secretion rate. The number of β-cells is derived from the cellular proliferation and death rates. Increasing functional β-cell mass could cure diabetes through pancreatic islet transplants or by strengthening endogenous cells. Various groups have shown that the β-cells proliferation rate is extremely low after adolescence in the majority of the population. However, β-cell proliferation has been shown to increase during physiological conditions such as pregnancy and obesity (1). This demonstrates that the molecular mechanisms that permit β-cell proliferation can be activated, given the right signals, after adolescence.
Nkx6.1, a β-cell transcription factor, was previously known to increase functional β-cell mass through induction of the genes Nr4a1, Nr4a3 and VGF (2, 3). Nr4a1 and Nr4a3 are sufficient to induce β-cell proliferation, and VGF is able to enhance glucose stimulated insulin secretion (GSIS). We have previously shown that Nkx6.1 induces c-Fos (Figure 1A), and that c- Fos induces VGF (Figure 1B). Therefore, our project goal was to determine if the c-Fos is part of the pathway by which Nkx6.1 increases β-cell proliferation, GSIS, and cell survival.
Results
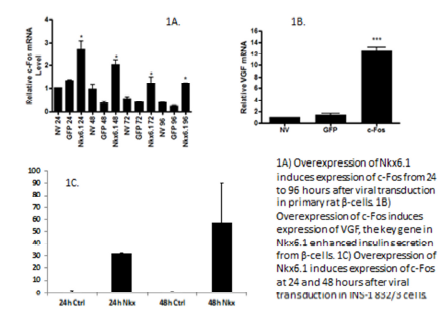
Nkx6.1 induces c-Fos in rat islets and β-cells.
Primary rat islets were treated with AdNkx6.1, AdGFP, or untreated. Samples were collected at 24, 48, 72, and 96 hours, and c-Fos mRNA was measured by RT-PCR. Results show that c-Fos is upregulated at 24, 48, 72 and 96 hours, with the strongest expression at 24 hours (Figure 1A). In addition, INS-1 832/3 cells were treated with AdNkx6.1 or AdGFP. Cells were harvested at 12, 24, 36, 48, 72, and 96 hours. Data shows c-Fos expression 48 hours after overexpression of Nkx6.1 (Figure 1C). These data demonstrate that Nkx6.1 induces expression of c-Fos in β-cells.
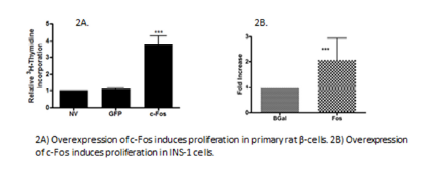
c-Fos causes proliferation in islets and β-cell.
Primary rat islets were treated with AdFos, AdβGal, or left untreated. Proliferation was measured by ³HThymidine incorporation. Results show that c-Fos induces proliferation in primary rat islets (Figure 2A). INS-1 832/3 cells were treated with AdFos and AdβGal. Cells were counted 96 hours after viral addition. Data shows that c-Fos induces proliferation in INS-1 cells (Figure 2B). These data demonstrate that c-Fos is sufficient to induce β-cell proliferation in β-cells.
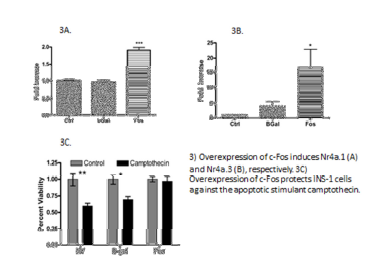
c-Fos induces Nr4a1 and Nr4a3 in rat islets.
Primary rat islets were treated with AdFos, AdβGal, or left untreated. Islets were harvested at 12, 24, 36, and 48 hours after addition of virus. Nr4a1 and Nr4a3 mRNA was measured by RT-PCR. Data shows that both Nr4a1 and Nr4a3 are induced by c-Fos (Figure 3A-B). These data demonstrate that c-Fos induces Nr4a expression, and suggests a mechanism by which c-Fos can drive β-cell proliferation.
c-Fos protects β-cells against apoptosis.
INS-1 832/3 cells were treated with AdFos, AdβGal, or left untreated. 48 hours after addition of virus, cells were treated with 0.125 μM camptothecin and compared to untreated cells. Following a12 hours incubation with camptothecin, cells were counted. These data show that c-Fos protects INS-1 832/3 cells against apoptosis (Figure 3C). VGF has been shown to protect β-cells from apoptosis. This data suggests a mechanism by which c-Fos is able to protect β-cells from apoptotic injury.
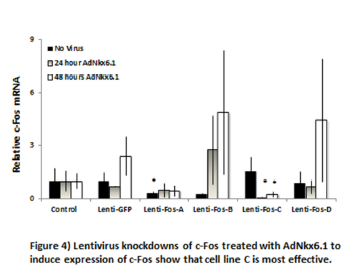
Lentivirus knockdown of c-Fos in INS-1 832/3 cells.
Lentiviral shRNA vectors were introduced to INS-1 832/3 cells to knock down c-Fos (4 varieties) and GFP. Lentiviral vectors also transferred resistance to puromycin. Puromycin selection pressure was applied until a parental INS-1 832/3 cell sample completely died. At this point, cells were treated with Nkx6.1 and harvested at 48 hours. c-Fos expression was measured by RT-PCR (Figure 4). These data show that the c-Fos knockdown C was most effective in decreasing Nkx6.1 induced expression of c-Fos.
Methods
Cell Culture and Reagents.
Male Wistar rats were purchased from Harlan and maintained on standard chow diet (Teklad 7001; Harlan). Rat pancreatic islets were isolated as follows: Briefly, the tissue was incubated with 20 ml of chilled Hanks’ balanced salt solution (HBSS) containing 0.5 percent collagenase (pH 7.25) at 38.5°C for 16 minutes with occasional vigorous shaking. The digestion was stopped by adding chilled HBSS containing 0.5 percent bovine serum albumin, and the digested tissue was washed three times in HBSS containing 0.5 percent bovine serum albumin. The final sediment was resuspended in 4 ml of 25 percent Ficoll in HBSS and was overlaid with 2 ml each of 23 percent, 20.5 percent, and 11 percent Ficoll (wt/wt). After centrifugation at 700×g for 15 minutes at 4°C, the islets were harvested from the 11 percent—20.5 percent Ficoll interface with a plastic pipette. The Ficoll was removed by washing the islets with HBSS containing 0.5 percent bovine serum albumin and centrifuging at 700×g for two minutes at room temperature. Rat studies were approved by the Brigham Young University Institutional Animal Use and Care Committee. Rat islets were cultured with AdNkx6.1, AdβGal, or AdFos.
[³H]Thymidine Uptake Assays.
[³H-methyl]thymidine was added at a final concentration of 1 μCi/mL to groups of ∼200 islets for the final 24–48 h of culture (depending on the experiment). Groups of 20 islets were picked in triplicate, washed twice in RPMI-1640 with unlabeled thymidine, twice in PBS, and then the DNA was precipitated with 500 μL of cold 10% trichloroacetic acid and solubilized with addition of 80 μL of 0.3 N NaOH. [³H]thymidine incorporation was measured by liquid scintillation counting and normalized to total cellular protein.
Proliferation in INS-1 832/3 cells.
INS-1 832/3 cells were plated at 2×105 cells/mL in 24 well plates. After 24 hours of incubation, no treatment, AdβGal, and varying concentrations of AdFos were applied for 4 hours. 72 hours after addition of adenovirus, cells were counted using a hemocytometer.
Apoptosis.
Cells were plated in 12 well plates at 2×105. After 24 hours, AdβGal, AdFos, and no treatment were applied for a period of 4 hours. After 48 hours the cells were exposed to the apoptotic stimulant Camptothecin at 0.125 μM for a period of 12 hours, then counted at approximately 60 hours from plating. Cell counts treated with camptothecin were normalized against cells not treated with camptothecin to give a fold decrease unaffected by c-Fos’s effect on cellular proliferation.
Quantitative RT-PCR.
Cells were harvested and placed in TRI-Reagent. mRNA was isolated according to standard procedure. mRNA was treated with Reverse Transcriptase kit and ran in PCR machine to synthesize cDNA. From cDNA, rat Nr4a1, Nr4a3, VGF, c-Fos, and PPIA were measured by quantitative RT-PCR.
c-Fos Knockdown.
Five lentiviral short interfering RNA vectors were introduced into samples of HEK 293t cells. Four vectors were different knockdowns of c-Fos, and the fifth was a standard knockdown of GFP. After cells had grown to confluency, media was harvested and added to INS-1 832/3 cells. Lentiviral vectors included resistance to puromycin. After maintaining cells in viral media, puromycin was added as a selection pressure until a control group of parental cells completely died. At this point, cell samples were treated with Nkx6.1, harvested, and c-Fos mRNA was measured by RT-PCR.
Discussion
Nkx6.1 has been shown to increase functional β-cell mass by inducing expression of Nr4a1/a3 (which induce proliferation) and VGF(which enhances insulin secretion and protects against apoptosis). Here, c- Fos is identified as the link between Nkx6.1 and the pathways controlled by the Nr4a nuclear receptors and VGF. This finding is significant for two reasons: first, because c-Fos comes on relatively quickly after Nkx6.1 is overexpressed, and second, because c-Fos controls both branches of the Nkx6.1 pathway, acting through both Nr4a1/13 and VGF.
Because of c-Fos’s early position in the Nkx6.1 pathway and its influence on genes that increase proliferation, decrease cell death, and increase GSIS, c-Fos is a potential drug target. Drugs targeting c- Fos could be used in one of two ways. First, c-Fos-inducing drugs could be applied to pancreatic islets prior to islet transplantation, resulting in highly efficient, resilient β-cells. This would allow for a decrease in the number of islet equivalents needed for transplant. Second, drugs that target c-Fos could be used to restore, increase, and maintain viable endogenous β-cell populations in diabetic individuals. This would avoid the potential problems of transplantation therapy by inducing a patients own β-cells to expand.
Conclusion
Additional studies to determine the role of c-Fos need to be done. We are currently determining the role of c-Fos on GSIS. We expect that c-Fos will enhance GSIS, given that it induces expression of VGF, which has been shown to enhance GSIS. In addition, we are interested in understanding if c-Fos protects against other inducers of apoptosis such as etoposide and thapsigargin. These experiments are currently ongoing.
In addition, we will use our c-Fos knock down β-cell line to determine the effect of c-Fos knock down on Nkx6.1 mediated proliferation, insulin secretion and protection from apoptosis. Specifically, we will confirm that Nkx6.1 mediated proliferation, protection, and GSIS occur through c-Fos, and that overexpression of Nkx6.1 in c-Fos knockdown cells is less effective in those respects than the parental cell line.
Works Cited
- Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167-76.
- Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, et al., cartographers. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.12008.
- Tessem JS, Moss LG, Chao LC, Arlotto M, Lu D, Jensen MV, et al. Nkx6.1 regulates islet betacell proliferation via Nr4a1 and Nr4a3 nuclear receptors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(14):5242-7.
