Jordon Ritchie and Steven Johnson, Department of Micro and Molecular Biology
Introduction
Nucleosome positioning plays an important role in gene regulation and expression. Nucleosomes consist of DNA-histone interactions that comprise the first order of DNA compaction into chromatin in the cell. Modifications to the histone in the nucleosome have been hypothesized to influence the location of the nucleosome on the DNA and therefore the regulation of the gene the nucleosome is forming on. In our original proposal, we proposed to show the effects that different modifications had on the position of the nucleosome on the DNA and the DNA sequence that had the highest affinity for nucleosome formation given a specific histone modification. A critical part of this experiment involves shearing the genomic DNA down to a functional size to work with. While planning our experiments we realized that the shearing process may not be random, but instead be a source of bias. In the event of a shearing bias, our nucleosome reconstitutions using the DNA fragments from our shearing would reflect the bias and potentially result in erroneous conclusions. Given this new question we realized that the randomness of DNA shearing needed to be addressed before we could proceed and thus we designed a series of experiments to determine whether or not DNA shearing was, in fact, random.
Methodology
In order to accurately determine whether or not shearing is random, we decided to use DNA from more than just our traditional model organism, the nematode worm C. elegans. Having multiple sources of DNA will give us variability in the DNA sequence across several genomes allowing us to examine potential bias in several organisms. DNA extractions from these model organisms use a standard phenol-chloroform extraction, relying on DNA’s hydrophilicity to separate it from inorganic phases during the extraction. The end step is an ethanol precipitation that causes the DNA to gather in a pellet that can be washed and resuspended for use in our shearing experiments.
Results
We have been able to recover high molecular weight DNA from these extractions indicated by a tight band near the top of the gel. Any visible smearing of the band indicates mechanical shearing that has already taken place during the course of the extraction. Ideally we would perform our shearing experiments on intact-high molecular weight DNA, but even DNA that has been mechanically sheared prior to our experiment is an interesting material to compare to non-sheared DNA.
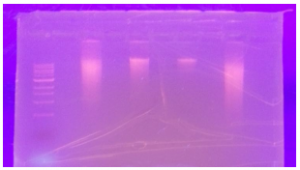
Discussion
We have thus far accumulated a substantial amount of DNA from our model organisms. The next step will be to determine the right size fragment to try and shear the DNA in preparation for sequencing. This will allow us also to hone the protocol for shearing down to the exact size we want to use going forward with the histone modification experiment. Whatever size DNA fragment we end up shearing to do the bias analysis will also be size fragment we use to do our reconstitutions with the histones. This way we will know the bias ahead of time and be able to accurately account for the bias in our data.
We are now in the process of shearing our DNA samples and will follow this with high-throughput DNA sequencing of the ends of the DNA molecules. Sophisticated bioinformatic analysis will allow us to definitively determine the randomness of DNA shearing and enable us to proceed with our original goal.
Conclusion
This data will also give us a better understanding of where the DNA is most likely to shear when it is under stress. Having this knowledge will better enable us to interpret our reconstitutions going forward as we try to see the effect of histone modifications on nucleosome modification and subsequent gene expression.
