Brandon Herrington and Jonathan Wisco, Department of Physiology and Developmental Biology
Introduction
As the 6th leading cause of death in the United States1, and because of the significant deterioration it causes in the quality of life of its victims, Alzheimer’s disease is an area where research to improve its early diagnosis is paramount to establishing new treatments. Alzheimer’s disease appears to show its effects at a particularly early stage within the hippocampus2. More specifically, the subicular area of the hippocampus is noted as a region that is subjected to more severe pathological changes due to the disease3. Braak staging is a method used to classify, into six stages, the degree of pathology in Alzheimer’s disease. By establishing a co-localization of iron to either tau or amyloid beta in the subiculum, iron could then be used as a biomarker for Alzheimer’s disease. This means that Magnetic Resonance Imaging (MRI) could potentially be used to detect tau and amyloid beta because of the signal dropout that iron induces in MRI. As a result, health professionals would then be able to use MRI to diagnose Alzheimer’s disease more effectively, and at an earlier stage—providing them the ability to establish a more effective treatment.
Method
Hippocampi of postmortem brains affected by Alzheimer’s were obtained. Each hippocampus was thinly sliced into several sections of seven microns in thickness, and placed on slides. Three slices from each hippocampus were then stained for tau, amyloid beta, and iron. After staining, detailed photographs were taken of these slides so that, for each hippocampus, we have a set of three high-resolution images, showing the deposit of amyloid beta, tau, and iron, all in essentially the same plane of the hippocampus.
The primary focus of our project was to combine each set of three images into one overlapping image that would clearly show the spatial correlation of amyloid beta, tau, and iron within the subiculum. We accomplished this goal by using Leica Virtual Imagine Software (Leica) along with Adobe Photoshop. In Leica, each image can be manually magnified and rotated to identical orientations, which gave us the ability to isolate the desired region of the hippocampus that we wished to examine—the subiculum in my case. After reaching the specific magnification and orientation of the image desired, we transferred these images to Photoshop. In Photoshop we targeted stained clusters of amyloid beta, tau, and iron in each image and changed their colors to green, blue, and red, respectively. These modified images were then superimposed to show a clear visual representation of how amyloid beta, tau, and iron co-localize.
Results
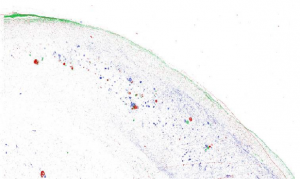
Our data set consisted of 5 hippocampi from deceased subjects: a 76-year-old (yo) F with cerebrovascular disease (CVD), AD Braak Stage VI and diffuse Lewy Body Disease; a 96 yo F with CVD and Braak VI; one 70 yo M with Braak VI only; an 86 yo M with Braak IV-V; and our control, an 81 yo F who suffered from scleroderma and severe pulmonary hypertension. A spatial correlation was observed between amyloid beta and iron in the subiculum of all four of our disease subjects. Tau, although showing an overlap with iron, was much more widespread throughout the subiculum in these four subjects (Figure 1). Our 81 yo F control did not show co-localization between iron, amyloid beta, or HP-tau.
Figure 1. A reconstructed image of a histological staining of the subiculum in the brain of our 96 yo F CVD/Braak VI subject. Iron is shown in red, amyloid beta is shown in green, and tau is shown in blue. Tau is distributed throughout the subiculum, while amyloid beta and iron appear to show more co-localization with each other.
Conclusion
Our lab previously demonstrated that in individuals with Braak VI, there exists a spatial correlation between only amyloid beta and iron in the hippocampus, but in the entorhinal cortex (found just adjacent to the subiculum), a co-localization between tau and iron only. This new data indicates that in the Braak VI subiculum, amyloid beta spatially correlates with only iron. This has sensitivity and specificity implications for diagnostic susceptibility weighted MR imaging. Our observation of widespread tau in the subiculum is consistent with the widespread tau usually observed in later Braak stages (V and VI). In future studies, our lab will continue to examine Braak stage V and VI hippocampi in order to further confirm the results that have been obtained thus far. Going forward, there should also be an attempt to explore the co-localization of tau with iron in earlier Braak stages.
1 National Center for Health Statistics, Centers for Disease Control and Prevention. (2010). Leading Causes of Death. Retrieved from http://www.cdc.gov/nchs/fastats/lcod.htm
2 Hampel, H., Burger, K., Teipel, S. J., Bokde, A. L., Zetterberg, H., & Blennow, K. (2008). Core candidate neurochemical and imaging biomarkers of alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 4(1), 38-48.
3 Falke, E., Nissanov, J., Mitchell, T. W., Bennett, D. A., Trojanowski, J. Q., & Arnold, S. E. (2003). Subicular dendritic arborization in alzheimer’s disease correlates with neurofibrillary tangle density. The American Journal of Pathology, 163(4), 1615-1621.