Steven L Castle, Department of Chemistry and Biochemistry
Introduction.
This report summarizes the results that were generated under the auspices of the mentoring environment in my laboratory from January 2013 to the present. A total of eight undergraduates participated in the mentoring environment during this period. Their names and accomplishments are listed below.
Evaluation of Academic Objectives.
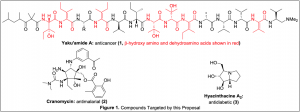
Our academic goals were to synthesize the organic compounds shown in Figure 1: yaku’amide A (1), cranomycin (2), and hyacinthacine A2 (3). Compound 1 is a peptide natural product that is toxic to cancer cells via a novel mode of action. Compounds 2 and 3 are naturally occurring alkaloids with activity against malaria and diabetes, respectively. Our purposes in synthesizing 1–3 were to (1) develop new chemical reactions that would be useful for us as well as for other researchers, and (2) use our synthetic route to prepare analogues for use in chemical biology studies designed to reveal the modes of action of the natural products. We have developed novel and efficient methods for synthesizing the unusual β-hydroxy amino and dehydroamino acids that are present in 1 (shown in red in Figure 1), and we are currently applying these methods to the construction of the natural product by linking the amino acids together to make larger building blocks. To date, this work has resulted in the publication of one paper with two undergraduate co-authors and one poster presentation by an undergraduate at a national scientific meeting. We have successfully fashioned the core structure of 2, and while we have not yet elaborated this intermediate into the natural product we have published one paper describing these results with two undergraduate coauthors. We are making strides toward the synthesis of 3, but these efforts are in their early stages and have yet to be published. We are still working toward accomplishing our academic objectives; nonetheless, we are pleased with our progress and the achievements that have resulted from this work.
Evaluation of Mentoring Environment.
Our mentoring goals are to train students in the techniques of organic synthesis, provide them with independent research projects, and prepare them for postgraduate education or employment. We have been successful in all three phases. During 2013 and 2014, four students who participated in this mentoring environment graduated from BYU. Two are currently Ph.D. students, and the other two are in medical school. Importantly, each of these students has been a co-author of a paper that was published in a prestigious peer-reviewed journal (see below).
The four students who are currently part of the mentoring environment all have plans to attend either graduate school or medical school. Clearly, students who graduate from this environment are achieving their goals. Moreover, promising new students are joining the environment, replacing the talented and hardworking researchers who have graduated. As a result, we believe that our mentoring goals are being achieved.
List of Students and Academic Deliverables.
- Bradley Naylor. Bradley successfully accomplished the synthesis of β-hydroxy amino acids via base-free aminohydroxylation. These compounds are key intermediates in the synthesis of yaku’amide A. He was a co-author of a paper that was published in a high-impact journal (J. Org. Chem. 2012, 77, 1208). Currently, he is a Biochemistry Ph.D. student at BYU.
- Phil Young. Phil assisted in the synthesis of the core structure of cranomycin. He was a co-author of a paper that was published in a high-impact journal (Org. Lett. 2013, 15, 1930). Phil graduated in April 2013, performed an internship at the Food and Drug Administration, and is currently a medical student at the University of Virginia.
- Joseph Cardon. Joseph worked toward the total synthesis of yaku’amide A. He presented a poster at the National Organic Symposium, which was held in June 2013 at the University of Washington. He was also a co-author of a recent paper (Org. Lett. 2014, 16, 4044). Joseph graduated in December 2013, performed an internship at a local polymers company, and began graduate school in chemistry at the University of California, Irvine this fall.
- Patrick Evans. Patrick worked with Phil on the cranomycin project and was a co-author of Phil’s Organic Letters paper. Patrick graduated in April 2014 and is currently a medical student at the University of Utah.
- Shi Luo. Shi joined the mentoring environment in Spring 2013. He is working toward the synthesis of yaku’amide A. He was a co-author of Joseph’s paper that was published in Organic Letters this year.
- Patrick Asay. Patrick joined the mentoring environment in Summer 2013. He is engaged in the synthesis of hyacinthacine A2.
- Brock Davis. Brock joined the mentoring environment in Winter 2014. He is working with Shi on the synthesis of yaku’amide A.
- Aaron Kubosumi. Aaron joined the mentoring environment in Winter 2014. He is developing new synthetic methods that are to be used for synthesizing hyacinthacine A2 and related compounds.
Description of Results.
We have developed a unified approach to the synthesis of the β-hydroxy amino and dehydroamino acids present in yaku’amide A (1). First, we perform an Os-catalyzed aminohydroxylation reaction (a process based on the J. Org. Chem. paper cited above) to generate β-hydroxy amino acids. Then, we conduct a stereospecific anti dehydration of these β-hydroxy amino acids to furnish the required dehydroamino acids. The recent Organic Letters paper co-authored by Joseph Cardon and Shi Luo describes this process. By using common precursors and a single synthetic route to prepare both β-hydroxy amino and dehydroamino acids, we should be able to synthesize 1 in a highly efficient manner. In the course of synthesizing cranomycin (2), Phil Young and Patrick Evans worked with graduate student Brad Loertscher to develop a method for the highly stereoselective formation of vicinal tertiary diols. This result is described in their Organic Letters paper that was published in 2013. To use this method to construct 2, we need to elaborate the core structure that we have prepared into the natural product by accomplishing an aziridination and a ring-opening reaction. In regards to hyacinthacine A2 (3), undergraduates Aaron Kubosumi and Patrick Asay have been working under the direction of graduate students Yu Cai and Ankur Jalan to explore the viability of an environmentally friendly iminyl radical cyclization process for generating a key subunit of this natural product. These studies are ongoing and should result in a publication during 2015.
Summary of How Funds Were Used.
The majority of MEG funds were used to pay salaries to the undergraduates involved in the research. Some MEG funds were used to support Yu Cai and Ankur Jalan, graduate students who have mentored Aaron Kubosumi and Patrick Asay. Some MEG funds were also used to purchase chemicals and other consumable supplies.
Conclusion.
Both 2013 and 2014 were productive years for our mentoring environment. We have yet to achieve our final goals of synthesizing yaku’amide A, cranomycin, and hyacinthacine A2. Nonetheless, we have published papers, presented posters, and laid the groundwork for future publications and presentations. We have learned much from the research funded by this MEG. Importantly, each of the eight students who participated in this environment has experienced personal growth and development as a scientist. It is extremely gratifying to have the opportunity to work with dedicated students and to help them reach their goals. I am grateful for the Mentoring Environment Grant program and the opportunities it has provided for me to work with outstanding undergraduates who are dedicated to learning.