Katalyn Pickett and Dr. John D. Bell, Undergraduate Education
Main Text
There are a number of ways that cells can die. One way is through apoptosis, also known as programmed cell death. This death pathway is characterized by orderly signals and mechanisms that essentially tell the cell to die without “making a mess”. Necrosis, another form of cell death, can be thought of as cell “homicide”; cells, generally in response to some environmental stress, are simply unable to survive and therefore die quickly and seemingly without regulation. Previously, the line between apoptosis and necrosis was thought to be distinct, but recent studies have shown that there are cell death pathways that share characteristics of both necrosis and apoptosis, and one of these, termed “necroptosis” (Degterev et al.), was of particular interest to me.
My hypothesis was that cells treated with thapsigargin (TG), a chemical meant to model endoplasmic reticulum stress-induced apoptosis, were also dying by necroptosis. I was led to this hypothesis by the results of previous experiments in which I inhibited caspases, proteolytic enzymes that are important in initiating apoptotic signaling cascades (Nicholson et al.), treated cells with TG, and assayed for important plasma membrane changes associated with apoptosis. Interestingly, around 50% of the cells still exhibited these changes, leading me to investigate other forms of cells death that are caspase-independent. In my research of the literature I found necrostatin-1 (Nec-1), a small molecule inhibitor of necroptosis. It has previously been shown to effectively rescue cells from necroptosis caused by a mechanism similar to that used by TG (Hsu et al.). I ordered it, and experimenting began.
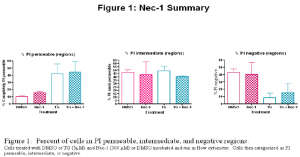
My experiments consisted of incubating S49 mouse lymphoma cells with 300 μM Nec-1 (or appropriate control) for 30 minutes prior to treatment with 5 μM TG (or appropriate control). The cells were then incubated for an additional 3 hours. After incubation the cells were washed according to a standard wash procedure, incubated with propidium iodide (PI), and run in a flow cytometer that measured the PI fluorescence intensity of each cell. Propidium iodide is a fluorescent probe, to which healthy cell membranes are impermeable, that fluoresces with greater intensity when bound to DNA. It is used as an indicator of membrane integrity because damaged membranes (those of dying cells) will allow PI entry into the cell and access to the DNA, and these cells will exhibit greater PI fluorescence intensity (Bailey et al.). Histograms of fluorescence intensity were generated and then analyzed using WEASEL data analysis software for flow cytometry. Cells were categorized as either PI permeable, PI intermediate, or PI negative. Results are shown in Figure 1.
In each of the categories t-tests were performed comparing treatment with and without Nec-1, none of which were significant (p>.05). These results indicate that Nec-1 has no significant effect on cells treated with TG, which leads to the conclusion that the cells dying independently of caspases are not utilizing the necroptotic death mechanism inhibited by Nec-1.
While these results were somewhat disappointing, they have led me to delve into other forms of cell death and inhibitors of cell death. I have begun a new project on an interesting and fairly new drug known as NecroX that is thought to inhibit certain forms of necrosis, and the preliminary results are promising.
Sources
- Bailey, R.W., Nguyen, T., Robertson, L., Gibbons, E., Nelson, J., Christensen, R.E., Bell, J.P., Judd, A.M., and Bell, J.D. (2009). Sequence of Physical Changes to the Cell Membrane During Glucocorticoid-Induced Apoptosis in S49 Lymphoma Cells. Biophysical Journal 96, 2709-2718.
- Degterev, A., Huang, Z.H., Boyce, M., Jagtap, P., Mizushima, N., Cuny, G.D., Mitchison, T.J., Moskowitz, M.A., Yuan, J.Y. (2005). Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chemical Biology 1, 112-119.
- Hsu, T.S., Yang, P.M., Tsai, J.S., and Lin, L.Y. (2009). Attenuation of cadmium-induced necrotic cell death by necrostatin-1: Potential necrostatin-1 acting sites. Toxicology and Applied Pharmacology 235, 153-162.
- Nicholson, D.W., Thornberry, N.A. (1997). Caspases: Killer Proteases. Trends in Biochemical Sciences 22, 299-306.