Hyrum Shumway and Dr. Steven Johnson, Microbiology and Molecular Biology
Introduction
Hershey and Chase proved DNA to be the genetic material of life in 1952. The structure of DNA largely eluded investigators until Watson and Crick discovered it a year later. These two landmark experiments laid a foundation so that questions in transcription, translation, gene regulation, and inheritance could be answered.
In recent years, a novel field of study has emerged, epigenetics. Epigenetics is the study of factors that effect gene regulation that do not stem from the primary sequence of DNA. The Johnson lab employs Caenorhabditis elegans as a model organism for this unique research.
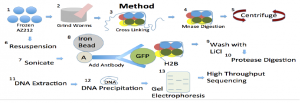
The goal of my project was to develop and validate a new protocol for tissue-specific isolation of nucleosomes through immunoprecipitation in young adult C. elegans. This protocol incorporates green fluorescent protein fused to histones to research nucleosome positioning in germ-line cells of C. elegans AZ212 nematodes. When successful, this protocol will allow analysis of chromatin architecture of any tissue at any developmental or disease state.
Methodology
Results

Shown above are the two gels showing the results of development and application of this new technique. Each gel shows DNA bands that have been run in an electric field and show DNA bands of the predicted size we sought to isolate. On the left each gel lane looks similar. There is no striking band that indicates that the correct nucleosomeal DNA was isolated. These pictures are what our lab saw dozens of times until I optimized our isolation protocol and produced the results on the right (Fig. 2).
Fig. 2 is the second success the lab saw in isolating the DNA. On the far right lane it is clear that there is DNA in between the 100 and 200 base pair markers. When we saw this we knew that we had isolated nucleosomal DNA. DNA wraps 1.67 times around the histone octamer making 147 base pairs in total being wrapped around the octamer forming the nucleosome. As this gel corroborated with our previous gel we had achieved a few days before we knew that the results were correct and that our protocol was working consistently.
Discussion
The results of this project have exceeded all expectations. In February of 2013 this project was presented at the Utah Conference of Undergraduate Research (UCUR). Later in early April this project was again presented at BYU for the Life Sciences Undergraduate Research Fair. However, at both of these events the research we had done up to that point had not achieved the research goal of isolating nucleosome associated DNA from C. elegans.
During April and May of 2013 we found two serious issues in our protocol. We had one reagent that had been confused with another as they have the exact same name but different chemical structures. Unbeknownst to us there had also been numerous chemical interactions that stifled the method we were trying to implement to isolate DNA. As we slowly discovered these issues and made significant changes to our protocol, we saw dramatic improvements that led us to produce figure 2 above.
Conclusion
The most exciting aspect of our research is that there is still so much to do. We have overcome a significant obstacle in getting the protocol to work. Now we need to utilize this protocol to other C. elegans strains and cell types.
Currently, our research is taking an exciting turn where we will use young adult C. elegans with our GFP antibody. This is a technique that has not been used elsewhere. One of the most exciting aspects of our unique research is that it can be easily applied in other cell types–including human cells. It also is a new way to characterize nucleosome positioning in multiple organisms and cell types. Previous studies that quantified nucleosome positioning employed nematodes in numerous life stages and did not screen for cell type. This study is highly specific and is restricted to young adult nematode germ line cells. No other labs utilize this method. The research we are doing currently has already opened collaboration with another university on this project. We anticipate that the protocol we have developed will lead to publishable results in the near term.
I would like to personally thank the Office of Research and Creative Activities for their generous support to me; I have benefitted immensely. This project has helped to prepare me for my eventual graduation. I feel more confident in going into the workplace with skills I have developed through the tutelage of Dr. Johnson. I also know that the research I have performed will be an asset to medical and scientific questions that face our society today. This research has already stretched me academically. Some of the issues we were able to unearth through careful diligence allowed me see the scientific method in action. I realize the importance of delving into the details at times to help solve issues in the larger picture. I am sure that this project will continue to push my creativity and skills as we seek to take the next steps in our research.