Charles Daniel Knechtel and Dr. Kim O’Neill, Microbiology
Macrophages are a part of the early immune system that interacts with abnormal and foreign cells in your body. They are a non-specific defense system that engulfs foreign cells or stimulates other immune system cells to respond. We know they can play a role in fighting cancer, or promoting it, but we don’t fully understand how. According to Frontiers in Bioscience May 2008, “At late stages tumor-associated macrophages are known to produce molecules directly promoting tumor growth, invasion and metastasis….however, if properly activated, macrophages may control initial tumor development”(Nardin, A., Astado, J. 2008). Cytokines are small signaling molecules that play an integral part in this interaction and the purpose of this study was to identify which are significantly altering our immune system’s ability to deal with cancer.
In order to better understand this, cancers of varying aggressiveness were grown in prescribed media and macrophages were extracted from whole blood using LSM (Lymphocyte Speration Media) and Optiprep® methods. Next, for 48 hours, macrophages and cancer cells were co-incubated together in filtered wells which stopped cells from touching but allowed signaling. Control samples of the cells were used to show changes in cytokines before co-incubation. After allowing the cells to communicate with each other, total RNA was extracted to see which genes for signaling were being expressed using an RNAqueous® kit. RNA was next converted to cDNA using Superscript III Master Mix®. The DNA was then prepped for RT-PCR using Syber Green® fluorescence, cytokine primers, and samples. RT-PCR is a method of amplifying DNA along with florescence, allowing researchers to quantify how much signal is being expressed and enabling comparisons. Mathematical calculations were analyzed using a Step One® comparative quantification program and the cytokine expression equation 2^-(Δct1- Δct2). Results were finally graphed to show the fold increase/decrease of significant signals.
Research subjects included the three breast cancers MDA-MB MCF7, 231, and 435, (MCF7 being the least and 435 being the most aggressive) along with the colon cancer HT-129. It has been shown that breast cancers have a large number of macrophages around the tumor that are not properly controlling the tumor environment (Almand et al 2000). Whereas macrophages associated with colon cancer appear able to infiltrate and to signal inflammation responses (Maeurer, M.J., Walter, W. et al. 1997). Perhaps there are different signals between breast and colon cancer that cause macrophages to act differently? LPS (Lipid Polysaccharides) were used as a control since it is a collection of bacterial membrane components which cause macrophages to become actively aggressive. Figure 1 below shows results of the most significant cytokine signals found.
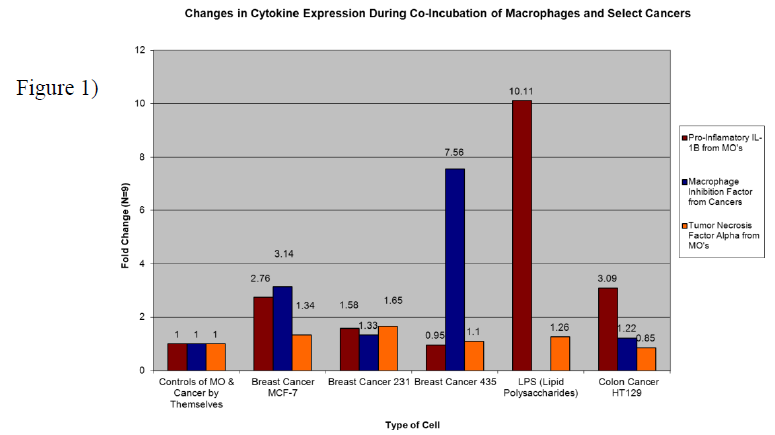
Red bars indicate an aggressive pro-inflammatory response signal from the MOs (macrophages) called IL-1b (Interleukin 1 beta). We see an expected increase of the signal when MOs are put with LPS. When MOs are placed with increasingly aggressive breast cancers we see a downward trend in expression levels. In fact, there is down-regulation of IL-1b when MOs are put with aggressive 435’s. This suggests that one reason for aggressive cancers being able to thrive is by causing MOs to down-regulate their IL-1b signals. Blue bars indicate MIF (Macrophage Migration Inhibition Factor) signals being expressed from the cancers. All the cancers increased their production of this signal when introduced to MOs but the most aggressive cancer (435) increased production by a very large amount, 7.56 fold more. Perhaps this signal is what is responsible for preventing macrophage infiltration into breast tumors. Colon cancer only increased MIF output very slightly (1.22 fold more) and with colon cancer MOs seem able to infiltrate properly. Orange bars represent MO expressed TNFa (Tumor Necrosis Factor alpha), a signal used by the immune system to help kill tumors through cell death and environmental inflammation. In contact with breast cancers 231 and MCF-7, MOs increased their signal output, but with 435’s there really was no change. An interesting observation is that colon cancer caused a decrease in MO expressed TNFa. Down-regulation of TNFa prevents MO’s from activating the cancer’s Caspase-8 pathway and causing cell death. The findings were presented as part of a poster on macrophage migration/response to breast cancers at the 101st AACR (American Association of Cancer Research) General Meeting in Washington DC April 20th, 2010.
The data suggests that breast cancer aggressiveness depends on the ability to inhibit macrophage mediated inflammation and movement (Il-1b & MIF). Colon cancer appears to act differently by inhibiting direct cell death signals instead (TNFa). Future treatment options need to utilize this understanding that different cancers inhibit the immune system through different signaling cascades. By preventing and up-regulating specific signals within tumor environments we may be able to help the body properly fight cancer.
Sources
- Almand, B., Resser, JR, Lindman B et al. 2000. Clinical Significance of Defective Dendritic Cell Differentiation in Cancer. Clinical Cancer Research. 6:1755-1766.
- Maeurer, M.J., Walter, W. et al. 1997. Interleukin-7 in Colorectal Cancer: IL-7 is Produced by Tissues from Colorectal Cancer and Promotes Preferential Expansion of Tumor Infiltrating Lymphocytes. Scandinavian Journal of Immunology. 45:182-192
- Nardin, A., Astado, J. 2008. Macrophages and Cancer. Frontiers in Bioscience. 13:3494-3505 Figure 1)
