Jared Kelson and Professor David Busath, Department of Physiology and Developmental Biology
Main Text
The reproduction of influenza A is very closely regulated by the pH levels maintained both in the cytoplasm and in various organelles of a host cell. To accommodate this, the virus provides genetic coding to assemble an M2 transporter which regulates the flow of protons across the different membranes. Until recently, influenza A was treated with a drug known commonly as amantadine (1-aminoadamantane), which blocked M2 proton transport. Unfortunately, by the year 2005, 91% of known influenza A strains had mutated to the extent that amantadine was no longer an effective reagent (High, 2006 p. 44). The 2009 epidemic of the H1N1 flu virus, more commonly known as swine flu, is a prime example of a mutated and unregulated strain reproducing freely in society, and demonstrates the ever increasing need to understand the method by which the virus can be stopped from propagating. My project began to test the amantadine resistance of various mutants which encode the influenza M2 proton transporter and localize the binding site of drugs which can potentially block it. Stopping this transporter will stop viral reproduction.
The M2 protein was grown in vivo through oocytes obtained from the African Clawed Frog, known scientifically as Xenopus laevis. Oocytes were harvested weekly, with great care taken in the recovery and maintenance of the frogs such that they remained healthy and without complication. The cells were then microinjected with 50 nL quantities of M2-encoded mRNA and allowed to incubate for 72 hours. Once the protein was fully expressed, the oocytes were impaled using a 2-electrode voltage clamp technique. The first electrode measured the membrane potential, naturally resting around -60 mV. The second electrode passed current into the cell, holding this potential stable and consistent when it would otherwise be altered by the changing conditions. Protons carry a positive charge, and upon entering the cell would raise this potential to a more positive value. As this influx was countered by the current provided through the second electrode, the change in current magnitude necessary to “clamp” the potential at a constant value served as a direct indicator of the number of protons entering through the M2 transporters. Throughout the experiment, the pH of the bath in which oocytes are held was acidified and the subsequent change in proton influx was recorded (see Mould, 2000 p. 31039).
The first strain M2 transporter was a wild type, and established the control by which to compare mutated strains. Figure 1 shows the functioning M2 transporter, followed by inhibition due to an amantadine block:
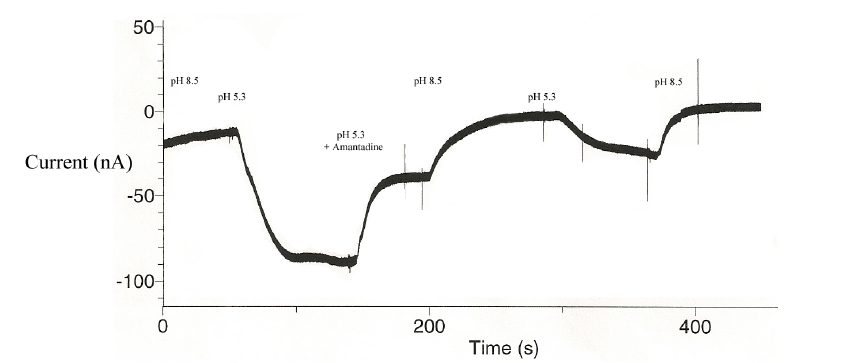
The actual mechanism by which amantadine can block the M2 transporter has been a topic of great debate,
with various contradictory conclusions (Khurana et. al., 2009; see also Schnell & Chou, 2008, Stouffer et. al.,2008). It has been observed that amongst the various new strains of influenza A, a very few amino acid sequences within the M2 gene are ALWAYS mutated. It is our hypothesis that such specific amino acids are therefore vital for the developed amantadine immunity. To test this, S31N was mutated by another branch of the lab, with the subsequent voltage clamp results shown below in Figure 2:
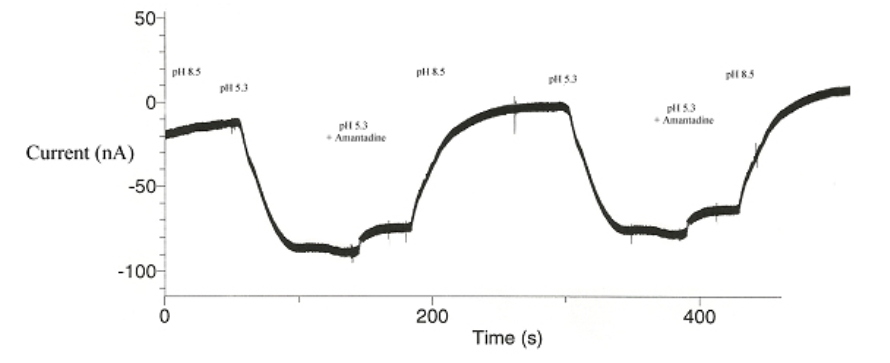
Since amantadine failed to block the influx of protons in the mutated strain, it is therefor suspected that the mutated serine (S31N) is vital to amantadine efficacy. These initial results were encouraging – we could accurately show that this point mutation interrupted amantadine’s ability to block M2 proton transport.
At this point I began testing this mutant with alternative compounds for new potential pharmaceuticals.
We had originally planned to publish our findings by the years end. However, due to unforeseen complications we were unable to reach this goal. One of the most important things I learned was the value of being a good team member. Oft times in research numerous people are involved in the team, and each is vital to the success of a project. I learned the necessity of accomplishing my tasks so that others in the lab could progress. I also learned to make time productive. At times progress was limited by other arms of the project, like obtaining the necessary RNA mutants for injection. While waiting for RNA to be synthesized, I was able to refine protocols and perfect the manual dexterity skills required to perform the various procedures of the project, effectively precluding future setbacks.
The next step in this project is twofold. First, potential M2-blocking compounds must continue to be tested. Second, other common mutations in influenza A must be incorporated, specifically D44N and D44A, to assess their role in amantadine resistance. This information will further our understanding of the M2 transporter and influence the list of compounds being compiled to replace amantadine as pharmaceutical reagents.
Sources
- “High levels of amantadine resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents ― United States, 2005-06 influenza season.” (2006, January 20). Center for Disease Control: Morbidity and Mortality Weekly Report. 55 (2): p44–6.
- Khurana, E., Peraro, M.D., DeVane, R., Vemparala, S., DeGrado, W.F., & Klein, M.L. (2009, January 27). Molecular dynamics calculations suggest conduction for the M2 proton channel from influenza A virus. PNAS. p1069-1074.
- Manor, J., Murkherjee, P., Leonov, H., Zanni, M.T., & Arkin, I.T. (2009, February 13). Gating mechanism of the influenza A M2 channel revealed by 1 and 2D spectroscopies. Structure. p247-254.
- Mould, J.A., Drury, J.E., Frings, S.M., Kaupp, U.B., Pekosz, A., Lamb, R.A., & Pinto, L.A. (2000, October 6). Permeation and Activation of the M2 Ion Channel of Influenza A Virus. Journal of Biological Chemistry. Vol 275, No. 40, p31038-31050.
- Schnell, J.R., & Chou, J.J. (2008, January 31). Structure and mechanism of the M2 proton channel of influenza A virus. Nature. p591-596.
- Stouffer, A.L., Acharya, R., Salmon, D., Levine, A.S., Costanzo L.D., Soto, C.S., Tereshko, V., Nanda, V., Stayrook, S., & DeGrado, W.F. (2008, January 31). Structural basis for the function and inhibition of an influenza virus proton channel. Nature. p596-600.
