David Holt and Robert Seegmiller, Department of Physiology and Developmental Biology
Introduction
In the present study, we report OA in a mouse model that, like the human families presented by Kannu et al., bears a heterozygous mutation in the Col2a1 gene but has a phenotypically normal skeleton.1 The mouse mutation was named sedc by Donahue et al. because when homozygous, it produces feature that resemble the most common clinical phenotypes of SED congenita in humans, including abnormal epiphyses, flattened vertebral bodies, and problems with the eyes and ears.2 The mutation consists of a single base pair substitution in exon 48 of the Col2a1 gene, which changes an arginine to a cysteine in the triple helical domain of the type II collagen protein. To look for signs of OA in the overtly normal heterozygous sedc (sedc/+) mice, we examined knee joint over the course of several months and found premature and progressive OA as demonstrated by loss of proteoglycans in the articular cartilage ECM, fibrillation of the articular surfaces, fissuring of the cartilage, and ultimately separation of uncalcified from calcified articular cartilage. Furthermore, we saw increased expression of HtrA1, a serine protease that is believed to degrade several different components of the PCM; of Ddr2, a cell surface receptor that binds native type II collagen as a ligand and upregulates MMP-13 in response; and of MMP- 13 itself, a matrix metalloproteinase that specifically digests type II collagen.3, 4 We conclude that the sedc/+ mouse is a useful model for OA in individuals with overtly normal skeletal structure who are predisposed to articular cartilage degeneration.
Methods
Wild type and sedc/+ mice at 2, 6, 9, and 12 months of age were euthanized by CO2 inhalation, and right knee joints were dissected and fixed in 4% paraformaldehyde overnight. Decalcification of each sample was confirmed through an ammonium oxalate reaction, and tissues were embedded in paraffin wax using an automated tissue processor. Sections were stained with Safranin O and fast green stains to visualize the degree of OA development. Immunohistochemistry was done on sections of mouse knee joints from every sixth slide from four wild type and four sedc/+ animals at 2-, 6-, 9-, and 12-month time points. Separate slides were stained for HtrA1, Ddr2, and MMP-13. Each slide was deparaffinized and then blocked for 1 hour. Primary antibodies against HtrA1, Ddr2, and MMP-13 were diluted to 1:200 concentrations, applied to specimens, and incubated overnight at 4o C. On the second day samples were rinsed with PBS and then incubated with an avidin/biotin ABC mix. Slides were rinsed a second time with PBS and incubated with biotinylated secondary antibody. After a third PBS rinse, a color reaction was initiated to achieve a red/brown stain using a peroxidase substrate. A cover slip was applied prior to photographing. Negative controls were prepared by staining without the addition of primary antibody.
Results
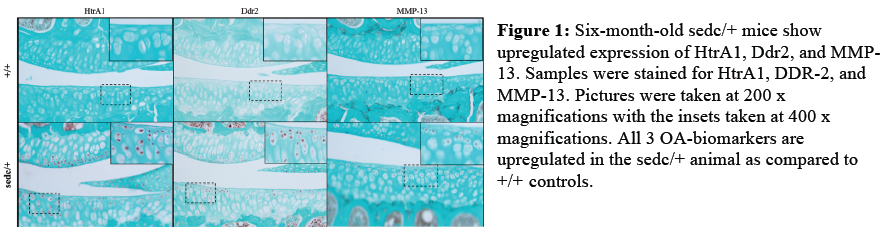
Immunhistochemical staining for HtrA1, Ddr2, and MMP-13 was virtually absent in knee articular cartilage from 2- and 6-month-old wild type controls. By 9 months, Htra1 staining began to appear in some wild type samples, and by 12 months, all three proteins were mildly elevated in some wild type animals. In sedc/+ knee samples, Htra1 and Ddr2 staining was detected at 2 months of age, but MMP-13 was not yet detectable. By 6 months, HtrA1, Ddr2, and MMP-13 were all markedly increased in sedc/+ samples (Figure 1). At 9 months, the expression of Ddr2 and MMP-13 continued to be elevated in sedc/+ compared to controls, but Htra1 levels were comparable to controls. At 12 months, only MMP-13 expression remained elevated compared to controls.
Discussion
The immunohistochemical results presented herein suggest that the articular cartilage degeneration we have observed in sedc/+ mice is consistent with the model proposed by Polur, et al.3 At 2 months of age, when only small regions of proteoglycan degredation in the superficial zone were apparent, HtrA1 and Ddr2 were detectable in sedc/+ knees. By 6 months, when proteoglycan degradation was more widespread and chondrocytes appeared to have increased their proteoglycan secretion to compensate, MMP-13 expression was increased as well. In wild type knee joints, HtrA1 upregulation was not observed until 9 months, and Ddr2 and MMP-13 elevation followed at 12 months of age. We have not directly tested cause and effect in this study, but it is interesting to note that the timing of HtrA1, Ddr2, and MMP-13 upregulation correlates with histologically detectable cartilage degradation in sedc/+ joints and is consistent with Polur’s proposed model.3
In conclusion, we submit that the sedc/+ mouse is a useful new model of OA. In addition, the sedc/+ mouse supports the contention that OA cases in the general population may be triggered or exacerbated by cartilage collagen mutations that have subtle effects on the structure of cartilage. An important focus in the field of OA research at present is determining whether the different forms of OA that develop as a result of genetic predisposition, chronic joint stress, or acute injury all follow the same molecular pathway of articular cartilage degradation once OA is initiated. The sedc/+ mouse will be useful to compare with chondrodysplasia models, surgical models, and other genetic models of OA to look for common molecular pathways, and ultimately, to develop novel therapeutic approaches that target those molecular pathways.
References
- Kannu P., Bateman J.F., Randle S., Cowie S., du Sart D., McGrath S., Edwards M., Savarirayan R.: Premature arthritis is a distinct type II collagen phenotype. Arthritis Rheum 2010, 62(5):1421-30.
- Donahue L.R., Chang B., Mohan S., Miyakoshi N., Wergedal J.E., Baylink D.J. et al: A missense mutation in the mouse Col2a1 gene causes spondyloepiphyseal dysplasia congenita, hearing loss, and retinoschisis. J Bone Miner Res 2003, 18(9):1612-21.
- Polur I., Lee P.L., Servais J.M., Xu L., Li Y.: Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histol Histopathol 2010, 25(5):599-608.
- Xu L., Peng H., Wu D., Hu K., Goldring M.B., Olsen B.R., Li Y.: Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem 2005, 280(1):548-55.
