Rachel Schneider and Dr. Jeff Edwards, Physiology and Developmental Biology
Introduction
My project goal was to understand how G-protein coupled receptor 55, (GPR55), a novel cellular pathway in the hippocampus, contributes to learning and memory. Recent research of neurodegenerative diseases, particularly Alzheimer’s, has primarily focused on understanding how learning occurs and how memories are encoded within the brain. Understanding the process in a normal brain and then how it is disrupted in a diseased brain is essential to making headway on treatments for neurodegeneration. Some studies have shown that to encode memories, the brain changes neural synapses to either strengthen or weaken the electrical pathways, which is a process known as synaptic plasticity. Since GPR55 is a protein receptor that has been shown to induce synaptic plasticity, understanding more about its function and role will help contribute to the understanding and possible treatments of neurodegenerative diseases. To answer the question of GPR55’s role in synaptic plasticity, I conducted electrophysiology experiments on rat hippocampal slices. I gathered several control experiments and attempted some experiments with a drug, LPI, which has been shown to enhance GPR55.
Methodology
Electrophysiology experiments are performed on the brain tissue of recently decapitated mice. In this particular experiment, the mouse is either a normal type that carries the gene for GPR55 (WT) or it is a mouse that has had the GPR55 gene knocked out (KO). The animal is terminated by being placed in a chamber of isoflurane gas which acts as an anesthetic. The animal is then decapitated and the brain is rapidly placed into an ice-cold, oxygenated medium containing essential ions. The brain is then adhered to a vibratome where it is cut into 400 μm coronal slices. This particular orientation maintains the hippocampal neural circuitry. The slices are then placed in a bath of room temperature oxygenated artificial cerebral spinal fluid solution (ACSF) containing necessary ions to mimic cerebral spinal fluid. The slices then must rest for at least an hour to perfuse oxygen within the tissues. After this time, the slices are submerged in a recording chamber which contains oxygenated ACSF at a temperature between 28-32oC. The ACSF submerges the slices continuously at a flow rate of 2-3 ml/min. A bipolar stimulating electrode is placed in the stratum radiatum, at least 400 μm from a recording electrode. This setup stimulates afferent neurons in the CA3 region. The excitatory post-synaptic potentials (EPSPs) are measured on an Axopatch 200B amplifier. To recreate long-term potentiation (LTP), or the strengthening of neural pathways, stimulation of the neurons in the CA3 region by the stimulating electrode is upped in frequency and magnitude at the beginning of each experiment for a short time (10-50 μA for 100 μsec at 0.1Hz sampling rate). EPSPs are then recorded for an additional 60 minutes to measure the effect of the LTP protocol on the brain, and to see if the neural pathway was strengthened. The data gathered from the electrode stimuli is then analyzed using Clampex and OriginLab software.
Results
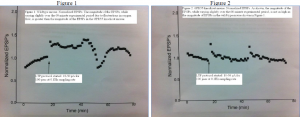
My project was designed to understand more about GPR55, a novel receptor that is thought to be involved in neural plasticity. While there is still much more to understand about GPR55, data gathered during my project helped solidify the idea that WT and GPR55 KO mice respond differently to LTP mechanisms. I was able to see a difference in experiments involving GPR55 KO mice versus WT mice, even though I did not previously know which type of mouse the slices came from. The differences of EPSP magnitude between GPR55 KO and WT mice is shown in Figures 1 and 2 below.
LPI, a drug which blocks the inhibitory pathway of the hippocampal neural circuitry, was also used in the experiments in order to see specifically the mechanism of GPR55 and if it is suspected to work through an excitatory pathway. However, the data I gathered on this topic was inconclusive. In many cases the slices simply did not respond well to the LTP protocol, which happens somewhat frequently in normal circumstances. Several more electrophysiology experiments will need to be done using LPI to understand more about the mechanism of GPR55 activity in neural plasticity.
Discussion
As expected, in my experiments, the GPR55 KO mice responses to neural pathway strengthening stimuli did not exhibit as much LTP as the WT mice. The magnitude of the EPSPs was much smaller for these GPR55 KO mice compared to the WT mice. A GPR55 KO is an excellent model to use when examining GPR55 specifically, as it is more effective than a GPR55 antagonist, or a drug that blocks GPR55 activity. Despite the effectiveness of using a knockout, the data from these experiments does not conclusively show how exactly GPR55 is involved in neural plasticity, but it is a step in the right direction. I regret that I was unable to gather data from more experiments, but many obstacles within the lab limited my ability to gather more conclusive data, including a stretch of a couple of months when the slices were simply not responding to stimuli normally. The project would be much better supported by more data, but the data I have gathered so far is a good start.
As previously mentioned, the drug LPI should be used to understand more about the specific mechanism of GPR55 in LTP. My experiments have helped to confirm the claim that GPR55 is involved in neural pathway strengthening mechanism, and future experiments in the lab will continue along this same vein.
Conclusion
There is still much to be understood about GPR55 and its role in neural plasticity. Further research will be done in Dr. Edwards’ lab to pin down the specific mechanism of GPR55 as a neural pathway strengthening agent. This information on specific mechanisms will be very useful in developing drugs or other therapies to both prevent and slow down the processes of neurodegeneration.