Austin Callison and Dr. William R. McCleary, Department of Molecular/Microbiology
Proposed Project
Just as simpler systems are studied to better understand more complex systems, E. coli has long been a standard for biological experiments aimed at a more complete comprehension of the mechanisms that propitiate cell life. One of these vital mechanisms in E. coli is the uptake of phosphate (Pi) in varying levels of environmental phosphorous. Pi is a key element involved in cell membranes, DNA, RNA, and metabolism in E. coli. This uptake is accomplished by the cooperation of the PstSCAB2, PhoU, PhoR and PhoB proteins in phosphate poor environments1. PhoU acts as a negative regulator of the Pho operon which regulates the expression of the aforementioned proteins1. The mechanism through which PhoU completes this function is still unknown1.
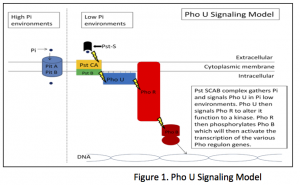
Previous studies show that PhoU is involved in a signaling pathway between PstSCAB2 protein and PhoR2. PstSCAB2, a group of periphery proteins, functions to sense the extracellular levels of Pi, scavenge the Pi and transport it across the cell membrane2. As the PstSCAB2 complex functions, it transmits a message via PhoU to PhoR. PhoR by default acts as a protein phosphatase, but when signaled by PhoU, transforms to function as a protein kinase, which will phophorylate PhoB4. Upon phosphorylation, PhoB functions as a promoter of the Pho regulon and activates transcription of the Pho proteins1 (see Figure 1). Through such cascade signaling, E. coli can maintain the proper levels of intracellular Pi necessary for cellular functioning when confronted with low levels of extra cellular Pi.
It has been hypothesized that PhoU functions primarily on or near the cell membrane, consistent with its involvement in signal transduction between PstSCAB2 and PhoR2. Our study involved the localization of the functional PhoU protein within E coli. This required the creation of a PhoU-GFP fusion protein and analysis through fluorescence microscopy. PhoU-GFP fusions initiating at both the amino- and carboxy terminal ends of PhoU have been constructed using standard PCR techniques thanks to the efforts of Mary Keller, a graduate student on rotation in our lab. Previous studies have found that other PhoU- GFP fusions render the protein nonfunctional1. With several stable ∆PhoU mutations created using promoter-swapping techniques3 in our lab, we were able to test our constructs for viability. With these viable PhoU-GFP fusion strains, we have been able to visualize the location of the functioning PhoU protein inside of E. coli.
Methods and Materials
With Mary’s PhoU-GFP fusions, we were able to conduct functionality experiments by screening for a downstream product of the Pho U signaling pathway. The results of these experiments showed a viable fusion that would mimic the activity of a normal E. coli. We were then able to test our fusion in various media. We prepared cultures with varying levels of expression of the PhoU protein in our different media. These cultures were then prepared to view via fluorescent microscopy. Photos were taken in an overlay fashion, one layer depicting the cells themselves, and the overlay layer depicting the fluorescing PhoU proteins. These photos display the location of the functioning protein inside of the E. coli cells.
Results
The fluorescent microscopy photos taken confirm that the functioning PhoU protein localizes at the periphery of the cells. This result can be seen as depicted in the following figure.
Figure 2-A depicts the E. coli cells in a high Pi MOPs solution grown for 24 hrs and viewed in simple white light microscopy at 100X. 2-B shows the fluorescence of the same cells under UV exposure and shows the cellular location of the functioning Pho U protein. As can be seen, the fluorescence it concentrated at the periphery of the cells and can be seen in the overlay in 2-C.
Research From Here
Though our hypothesis has been confirmed by the experiments and microscopy performed, further investigation into the mechanism of the Pho U protein is worth deliberate investigation. Preliminary work with quadruple PhoU- GFP mutants could have been done. Further work with these strains could shed further light on the particular mechanism of Pho U. The PhoU-GFP fusion opens the doors to many other enlightening experiments.
Sources
- Baek, Jong Hwan., Kang, Yeon Jae., Lee, Sang Yup. 2007 ‘Transcript and protein level analyses of the
interactions among PhoB, PhoR, PhoU and CreC in response to phosphate starvation in Escherichia coli.’ FEMS Microbiology Letters 277:254-259 - Muda, M., N. N. Rao, and A. Torriani. 1992 Role of PhoU in phosphate transport and alkaline phosphatase regulation. Journal of Bacteriology 174:8057-8064
- Rice, Christopher D., Pollard, Jacob E., Lewis, Zachery T., McCleary, William R. 2009 ‘Employment of a Promoter-Swapping Technique Shows that PhoU Modulates the Activity of the PstSCAB(2) ABC Transporter
in Escherichia coli.’ Applied and Environmental Microbiology 75:573-582 - Wanner, B. L. 1996 Phosphorous Assimilation and Control of the Phosphate Regulon, p. 1357-1381. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed. ASM Press, Washington, D.C.