Jace Bullard and Dr. Marc Hansen, Department of Physiology & Developmental Biology
Abstract
A major difficulty of treating cancer rises from its ability to metastasize. During metastasis, strong adhesions between cells break, allowing individual cells to separate and migrate to different locations in the body. Strong cell-cell junctions are formed by protein interactions between actin cables and cell junction proteins. Zyxin is an actin regulator that localizes at cadherin-based cell-cell adhesions. Our lab has shown that cells expressing constitutively active forms of zyxin lose the ability to completely detach from each other, a result of maintained actin-membrane associations. How zyxin is localized to the cell membrane is unknown. Given zyxin’s role in maintaining cell-cell adhesions, understanding the molecular basis of this function could provide new insight into how cells initiate metastasis. Based on previous research in our lab, we have proposed a role for nectin in localizing zyxin to cell-cell adhesions. We have carried out many experiments and gathered data that has required us to significantly change our approach.
Introduction
During cancer metastasis, individual tumor cells break free from the primary tumor and migrate to various locations in the body. This process mimics epithelial-mesenchymal transition (EMT), a process used to generate new tissues in development. In order for metastasis to occur, cells must dissociate junctions that bind them to one another. These junctions consist of adhesion protein complexes that bind to the actin cytoskeleton inside the cell.
Zyxin is a protein that localizes at adherens junctions. Zyxin binds to the vasodilator-stimulated phosphoprotein (VASP), which drives actin filament formation and binding (Drees, et al, 2000). This zyxin-VASP-actin connection generates points of strong adhesions between cells, preventing their dissociation (Sperry, et al, 2010). Elucidating how zyxin-VASP-actin connections are linked to the cell membrane will reveal how cells normally prevent detachment and provide clues as to how cells initiate metastasis. This would allow for development of new strategies to prevent cancer spread. Factors responsible for localizing zyxin to the junctions are unknown. Results from our lab indicate that zyxin is localized to adherens junctions independently of actin, and that amino acids 120-280 are essential for zyxin’s localization to the membrane. A yeast-two hybrid assay was performed in our lab using zyxin fragment containing this region as bait and several potential binding partners were revealed. Among these is nectin, a well-known transmembrane protein. Nectin proteins dimerize with nectins on neighboring cells and are components of adherens junctions. Down- regulation of nectin has been shown to occur in certain metastatic cancers (Guzman, et al, 2006). Nectin has also been linked to the actin cytoskeleton through the nectin and actin binding protein afadin (Takai, et. al, 2003).
Nectin’s roles in cell adhesion and cancer metastasis, as well as its affiliation with adherens junctions and actin make it a premium candidate for localizing zyxin to the cell membrane. Based on the preliminary yeast two hybrid results, our hypothesis is that nectin is responsible for localizing zyxin at adherens junctions. Discovering this relationship will shed further light on the pathways of cancer metastasis.
Materials and Methods
Cell Lines: We used MDCK, L, and LE cells. L and LE cells are derived from mouse fibroblasts and exhibit no cell-cell adhesion. All cells cultured in DMEM media.
Transfections were performed with QIAGEN Effectine transfection kits on 70,000 cells.
Plasmids created using dsRed, pQe and pGEX 6p1 vector backbones. Plasmids were transformed into e. coli. competent cells, harvested via midi-prep and transfected into mammalian cells.
Antibodies for staining were monoclonal IgG for e-cadherin, afadin, and zyxin.
Results/Discussion
Specific Aim #1: Show that nectin localizes zyxin to cell-cell adhesions
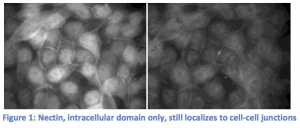
Our experiments to show that zyxin localization relies on nectin have changed drastically from those that were originally planned. We started by transiently transfecting MDCK cells with the intracellular domain of nectin only fused to a fluorescent tag. We wanted to see that our DNA construct was working correctly, but found that nectin was still localizing at cell-cell junctions (Figure 1). In considering possible explanations for nectin’s localization to the cell
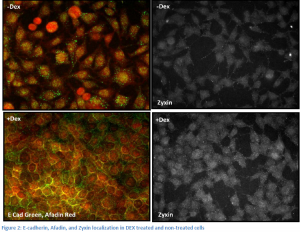
membrane without it’s trans-membrane domain, we realized that nectin may be binding to other proteins associated with the cell membrane like afadin, alpha catenin, and e-cadherin. We decided to control these factors by using a line of L-cells (cells lacking any cell-cell adhesion) carrying a chemically inducible plasmid containing an e-cadherin gene. These cells, LE cells, express e-cadherin upon treatment with dextramethasone (DEX). We planned to transfect LE cells with nectin, and stain for zyxin and afadin, a protein known to bind nectin. We already possessed an afadin antibody and chose to stain for it rather than nectin to save time and money. In transfected cells induced by DEX, we expected to see the formation of cell adhesions, with afadin (nectin), zyxin, and e-cadherin all co-localized at cell junctions. In non-transfected, DEX treated cells, we expected to see e-cadherin at the junctions, but afadin and zyxin diffuse within the cells. Upon completion of these experiments, however, we observed conflicting results. Indeed, when the cells were induced with DEX, cell-adhesions formed and e-cadherin and afadin moved to the cell junctions. However, afadin moves to the membrane upon DEX treatment regardless of the cells being transfected with nectin. In addition, zyxin did not appear at the junctions in either case (See figure 2).
The only way these results can be explained is by the endogenous expression of nectin in LE cells and a mistake in our zyxin staining. Upon further investigation, we found that a form of nectin, nectin 2, is in fact expressed in L and LE cells. Now, our experiments rely on knocking down expression of nectin rather than inducing it. We purchased nectin-2 shRNA and nectin-2 antibodies. We have been perfecting transfection of LE cells with the shRNA in order to determine which vector induces the most complete knockdown of nectin-2 expression. Once we establish a good knockdown, we will transfect DEX treated and non-treated LE cells with the shRNA and stain for nectin-2, e-cadherin and zyxin. We expect to see zyxin localization at the cell membrane perturbed upon shRNA knockdown of nectin-2. In DEX treated cells without shRNA knockdown, we expect to see nectin-2, zyxin, and e-cadherin co-localized at cell junctions.
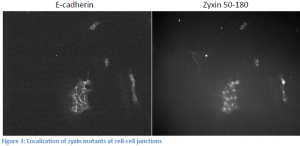
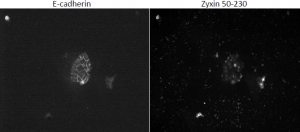
We have lost significant time due to problems with tissue culture. We have had rampant infections that destroy the cells, and progress has been slow. Due to this fact, we have taken on a side project related to but not mentioned in my original proposal. The exact domain responsible for zyxin’s localization to the cell membrane has not been elucidated. We know that the domain responsible for bringing zyxin to cell membranes is between amino acids 120 and 280. We created two zyxin mutants, one encoding amino acids 50-180, and the other 50-230 both fused to fluorescent tags. We transfected MDCK cells with each plasmid and stained for e-cadherin in order to locate cell-cell junctions. Upon visualization, the 50-180 fragment appeared to localize at the membrane, while the 50-230 fragment did not (see figure 3). These results are contradictory. We expect the 50-180 fragment, which expresses less of the protein, to not be able to localize to the cell membrane. We are in the process of recreating mutant cell lines and will re-stain. We will also test our DNA to ensure the appropriate DNA is being transfected. Once we obtain good results, we can create more zyxin mutants and effectively “widdle-down” zyxin until we isolate the exact domain responsible for its localization. Presumably, this is the domain that binds nectin.
Specific Aim #2: Show that nectin and zyxin interact directly
In order to prove a direct interaction between nectin and zyxin, we originally planned to perform pull-down assays. Nectin was produced and purified with no problems, but we could not produce zyxinΔlim ( a constitutively active zyxin mutant). It took two weeks to re-make the zyxinΔlim DNA plasmid, and after its completion we were able to produce and purify our zyxin mutant. However, this new mutant was inserted into a vector that attached a GST tag to our protein. We were unsuccessful at inserting the mutant into a His-tag encoding vector. As our nectin construct was also GST, we could no longer perform pull-down assays due to the potential for confounding GST-GST interactions. We then decided to move to sucrose gradient binding assays instead of pull-downs, in which these confounding interactions are not a factor. The gradients were set up and when the fractions were run in gels, unusual bands corresponding to a high molecular weight were observed. We concluded that these bands were the result of concatamerization, a phenomenon that can occur between the head and tail binding domains of separate zyxin molecules. Since a band corresponding to nectin’s molecular weight was absent, we assumed that nectin was also bound to the zyxin concatamers. If this observation could be confirmed, our hypothesis of nectin and zyxin’s direct interaction would have been shown to be valid. We made an attempt to repeat the sucrose gradient assay, but gel analysis of the re-purified proteins led us to believe that the band we thought was representing nectin was actually a contaminant also showing up in our previous sucrose gradient assay. A third repeat of the experiment was similarly inconclusive, and subsequent attempts to confirm a purification of nectin have failed. We suspect that nectin is degrading rapidly, so have ordered several nectin types from a lab in Japan that will allow us to perform our initial pull-down assays.
Conclusion
In conclusion, it is still unclear whether nectin is responsible for localizing zyxin to cell-cell adhesions. We have gone through significant troubleshooting phases, and now that we have a more trustworthy approach, we expect to obtain conclusive results within the next month.
Acknowledgements
BYU Cancer Research Center
Dr. Marc Hansen
John Davis
Jarek Atwood
Dave Reeder
References
- Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, Golsteyn RM. (2000) Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J Biol Chem. 2000 Jul 21;275(29):22503-11.
- Guzman, G, Oh, S, Shukla, D, Valyi-Nagy, T. (2006) Nectin-1 Expression in the Normal and Neoplastic Human Uterine Cervix. Archives of Pathology & Laboratory Medicine; Aug2006, Vol. 130 Issue 8, p1193-1195, 3p
- Moody JD, Grange J, Ascione MPA, Boothe D, Bushnell E, and Hansen MDH. (2009) A zyxin head-tail interaction regulates zyxin-VASP complex formation. Biochem. Biophys. Res. Comm. 378: 625-628
- Sperry RB, Bishop NH, Bramwell JJ, Brodeur MN, Carter MJ, Fowler BT, Lewis ZB, Maxfield SD, Staley DM, Vellinga RM, Hansen MD. (2010) Zyxin controls migration in epithelial-mesenchymal transition by mediating actin-membrane linkages at cell-cell junctions. J Cell Physiol. 2010 Mar;222(3):612-24.
- Takai Y, Nakanishi H. (2003) Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003 Jan 1;116(Pt 1):17-27