Amanda Berbert and Dr. John Bell, Department of Physiology and Developmental Biology
Overview
Our project provided us the opportunity to gain insight into how chemotherapy drugs affect the cell membrane and how secretory phospholipase A2 (sPLA2) can work in tandem with these drugs to accelerate the cell death process. The enzyme sPLA2 produces a pro-inflammatory response that plays an important role in wound protection and healing and is also associated with diseases including septic shock, atherosclerosis, and cancer. This enzyme preferentially hydrolyzes cells that exhibit specific physical membrane properties in response to cellular trauma or apoptosis (programmed cell death). We hypothesized that although chemotherapeutic agents work through different mechanisms, the drugs induce similar biophysical membrane changes. Therefore, we expected sPLA2 to increase the hydrolysis of our cancerous cell membranes after they are treated with chemotherapeutic agents irrespective of the inducer. This would suggest a role for sPLA2 as a therapeutic ally during chemotherapy.
Our Experiments
For our project, we used fluorescent probes to assay the biophysical changes that occur at the cell membrane after the addition of different chemotherapeutic drugs. Then we related those changes to the amount of hydrolysis by sPLA2. We used four different chemotherapeutic drugs: paclitaxel (Pac), actinomycin-D (Act-D), methotrexate (MTX), and daunorubicin (DNR). After incubating S49 lymphoma cells with one of these drugs for a specific length of time, we measured the changes which occurred at the cell membrane. For each drug, we chose an early, middle, and late time point of apoptosis in order to assess how the cell membrane changes over time during the death process. At each time point, we used the fluorescent probes propidium iodide (PI), merocyanine (MC540), trimethylammonium diphenylhexatriene (TMA-DPH), and diphenylhexatriene (DPH) to assess individual membrane properties.
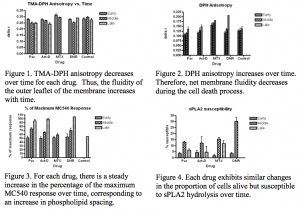
First, we assessed membrane fluidity by activating TMA-DPH or DPH with plane-polarized light in a spectrofluorometer. When the membrane is in a more rigid state, more of the light emitted from the probe remains in the polarized state at which it was excited, whereas when the membrane is in a more fluid state, a smaller fraction of the light remains in the same orientation. At early time points, we observed high anisotropy readings (light remains in mainly the same rotation) when TMA-DPH was used as our probe and low anisotropy readings when DPH was used, as shown in Figures 1 and 2. Because the charged acidic group on TMA-DPH confines this probe to the outer leaflet of the membrane, we interpreted the decreasing anisotropy values of this probe as evidence that lipid order decreases and membrane fluidity increases with time.
In contrast, because DPH is uncharged and is therefore capable of entering the intracellular space, we interpreted the increasing anisotropy readings detected with DPH to indicate a net increase in lipid order and a net decrease in membrane fluidity in the cell when all membranes were taken into consideration.
Next, we examined changes in interlipid spacing. The probe MC540 allowed us to assess phospholipid spacing since its fluorescence intensity increases as the spacing between phospholipid head groups increases. We integrated the spectra for the interval of recorded intensity up to 615nm and graphed the percentage of the maximum MC540 response versus time. We observed a steady increase in the percentage of the maximum response with time since addition of the drug (Fig. 3), which correlates with an increase in interlipid spacing.
Lastly, we used a spectrofluorometer to detect the fluorescence intensity of PI. The fluorescence of PI drastically increases when the cell membrane is compromised and PI binds DNA. This allowed us to assess cell susceptibility to hydrolysis and determine the proportion of cells which were alive but susceptible to hydrolysis by the sPLA2 enzyme at each time point (Fig. 4).
Conclusion and Future Work
Remarkably, the results of our spectrofluorometer experiments were similar for each chemotherapy drug which we tested. These results are interesting because they show that each drug produces similar alterations to the cell membrane, even though each drug induces cell death through a unique mechanism. Further, sPLA2 increased the hydrolysis of the cell membrane after addition of any of the four chemotherapeutic drugs we used. We look forward to continuing the data analysis phase of our work and aim to publish our findings in a scientific journal in the near future. Our lab is also exploring whether our findings our reproducible in human lymphocytes.
In addition to the scientific knowledge we gained, our project also allowed us to learn about the research process and how to deal with challenges that arise along the way. For example, the unanticipated difficulties we faced while trying to interpret some of our data gave us practice in critical thinking. We also learned the importance of planning ahead and coordinating our experiments with our mentor and other lab members. We are sincerely grateful for this research experience and know it will be invaluable in our future careers.