Michael Baldwin and Dr. Laura Bridgewater, Department of Microbiology and Molecular Biology
Introduction
More than 40 million Americans suffer from the pains of arthritis. Arthritis is characterized by joint inflammation and cartilage deterioration (3). For many years growth differentiation factor 5 (Gdf5) has been associated with cartilage formation and maintenance (4, 1). GDF5 has been traditionally regarded as a secreted growth factor; however, a new variant of Gdf5 was recently discovered in the nucleus. Nuclear Gdf5 (nGdf5) may present a new mechanism by which Gdf5 regulates cartilage development and preservation.
Preliminary Research
Nuclear Gdf5 (nGdf5) was first found while studying enhancer elements of the Col11a2 gene. A yeast-one hybrid screen suggested that Gdf5 can bind to the D/E enhancer of this gene (2). Further experiments confirmed the presence of nGdf5 in the nuclei of cells and determined the specific mechanism by which nGdf5 is translocated to the nucleus (2). This work led to the hypothesis that nGdf5 functions as a transcription factor, binding to regulatory regions of target genes to modulate transcription.
Objective
The long-term purpose of my research is to learn the specific function of nGdf5 and to determine its regulatory pathway. The goal of this project was to identify a consensus DNA binding sequence for nGdf5. Understanding a specific nGdf5-DNA interaction would pave the way for future work examining the potential role of nGdf5 as a transcription factor.
Methods and Results
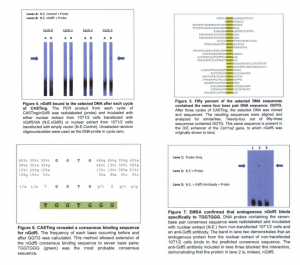
Cyclic amplification and selection of targets (CASTing) was performed to identify a consensus DNA binding sequence for nGdf5 [fig. 3]. An electrophoretic mobility shift assay (EMSA) was performed after each cycle of CASTing to verify nGdf5-DNA interactions [fig.4]. After three cycles of CASTing, the selected DNA was cloned and sequenced. The resulting sequences were aligned [fig.5] and analyzed to identify a consensus sequence [fig.6]. To confirm that nGdf5 binds to the resulting consensus sequence (TGGTGGG), probes containing TGGTGGG were synthesized and utilized in an EMSA with nuclear extract from non-transfected 10T1/2 cells [fig.7].
Conclusion
My research demonstrates that nGdf5 binds specifically to the consensus sequence TGGTGGG. Further experiments will be conducted to determine whether nGdf5 functions as a transcription factor. Specifically, genes that contain TGGTGGG in their regulatory regions will be identified, and luciferase reporter plasmids containing these regulatory regions will be constructed. Co-transfection of nGdf5 with each reporter plasmid will reveal whether nGdf5 is capable of binding to TGGTGG-containing regulatory regions to alter gene expression. Understanding nGdf5’s role as a transcription factor may provide new information on cartilage breakdown and may establish new means for treating arthritis.
References
- Chhabra, Anikar, et al. “BMP-14 Deficiency Inhibits Long Bone Fracture Healing: A Biochemical, Histologic, and Radiographic Assessment.” JOT 19 (2005): 629-634.
- Felin, Jenny E., et al. “Nuclear Variants of Bone Morphogenetic Proteins.” BMC Cell Biology 11:20 (2010).
- “Living with Arthritis.” JAMA 282 (1999): 1982.
- Rountree, Ryan B., et al. “BMP Receptor Signaling Is Required for Postnatal Maintenance of Articular Cartilage.” PLOS Biology 2 (2004): 1815-1827.