Tobin Story and Dr. Bryan Hopkins, Plant and Wildlife Sciences
The purpose of this research was to gain a better understanding of the factors affecting emissions of nitrous oxide and ammonia from fertilized agricultural soils. Nitrogen is an essential nutrient required for sustaining life and high-output cropping systems. However, it has been estimated that only 40-60% of applied fertilizer N is utilized by plant-uptake. Nitrogen that is not utilized by plants can be lost to the environment in a variety of ways, two of which are of particular concern. Denitrification of nitrates produces nitrous oxide, which is a proven greenhouse gas with a long atmospheric lifetime and a heat trapping capacity roughly 296 times that of CO2. Agricultural production contributes 81% of total anthropogenic N2O emissions worldwide, making it by far the largest contributor. Second, urea fertilizer applied under certain conditions can quickly be volatilized to ammonia gas (NH3). Increased atmospheric NH3 concentrations have been linked with haze, eutrophication, and forest decline.
We focused specifically on the differences between the current standard practice, which is the application of a urea-based nitrogen fertilizer, and a modified practice that uses a slow-release, polymer-coated urea. It was hypothesized that the slow-release fertilizer would result in significantly reduced emissions of both nitrous oxide and ammonia gas. After conducting preliminary research, it was determined that the best course of action would be to attempt a continuous measurement of gaseous emissions in a semi-enclosed system. We constructed a system of chambers, each containing an appropriate amount of loam soil and several maize (Z. mays L.) plants in a ventilated chamber. Each chamber was attached via tubing to a multiplexer, which was in turn attached to a field gas monitor.
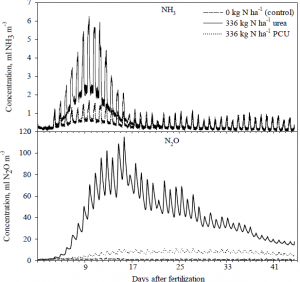
After construction of the chambers, fertilizer was applied as urea, polymer coated urea, or not applied as a control. Gaseous emissions were monitored continually for 45 days. As hypothesized, the use of polymer-coated urea significantly reduced the emissions of both nitrous oxide and ammonia. In fact, nitrous oxide emissions were reduced by almost 90% when compared to urea, and ammonia emissions were reduced by more than 50%. In addition to proving our hypothesis, we were also able to achieve a better understanding of the daily cycles of both nitrous oxide and ammonia emissions.
Previous experiments were unable to sample more than once or twice daily, and therefore suffered from low temporal resolution – they chronicled only a snapshot of the actual cycle. With the continuous monitoring capabilities of our system, we were able to see the full picture of soil-gas emission cycles. This may prove important because current estimates of agricultural greenhouse gas emissions may be on the low end of the spectrum due to sampling error.
The results from this research were published as a part of a thesis submitted by Joshua J. LeMonte, entitled, “Environmental Implications of Polymer Coated Urea.” In addition, the findings from this research were presented at both the annual meetings of the Soil Science Society of America and the National Conference on Undergraduate research under the title “ Polymer Coated Urea: Reduction of Ammonia Volatilization.” Attached is a graphic showing the concentration of gases in the headspace of our sampling chambers. This graph represents the averages of 4 chambers each for polymer coated urea, urea, and control treatments.
References
- Joshua J. LeMonte; Assessing Atmospheric Losses of Nitrogen with Photoacoustic Infrared Spectroscopy: Urea vs. Polymer Coated Urea; Figure 3