Jason Porter and Dr. Paul Reynolds, Physiology and Developmental Biology
The study of developing tissues is extremely important in the field of biology. Each organ in the body adheres to an extremely complex and organized development process involving specific signaling molecules and pathways. Pulmonary development is no exception. Lung development adheres to intimately orchestrated processes that require precisely regulated reciprocal interactions between developing respiratory epithelium and the surrounding splanchnic mesenchyme. Proper lung development involves both spatial and temporal control of a myriad of factors including transcription factors, growth factors, cell surface receptors, and extracellular matrix constituents. My hypothesis when I began this project was that 5 subunits are expressed in specific lung cell types such as Clara cells in the proximal lung and alveolar type 1 (AT1) cells in the distal lung. I further hypothesized that 5 containing receptors function in signaling during normal development.
5 nicotinic acetylcholine receptor (nAChR) subunits are structural subunits that stabilize functional nAChRs in many non-neuronal tissue types. To determine possible functions of 5 nAChR subunits during lung morphogenesis, the expression of 5 and cell-specific markers were assessed by co-localizing immunohistochemistry from embryonic day (E) 13.5 to post natal day (PN) 20.
In the results of my experiments I observed a steady expression of 5 nAChR subunits in distal lung epithelial cells during development while proximal lung expression significantly alternates between abundant prenatal expression, absence at PN4 and PN10, and a return to intense expression at PN20. 5 expression was most abundant on luminal edges of alveolar type (AT) I and ATII cells, non-ciliated Clara cells, and ciliated cells in the proximal lung at various periods of lung formation.
The data I collected reveals a highly regulated temporal-spatial pattern of 5 nAChR subunit expression during important periods of lung morphogenesis. Due to identification of 5 in alveolar epithelium and Clara cells, future studies may identify possible mechanisms of cell differentiation and lung homeostasis mediated at least in part by 5-containing nAChRs.
This research experience was invaluable. I was not only able to conduct interesting experiments in my field of study with a knowledgeable and helpful mentor but I was also able to present my research at a national meeting and publish my research in a peer-reviewed journal. I presented my findings at the national Experimental Biology meeting in Washington, D.C. with other colleagues from my lab. At this meeting I was not only able to learn more about new future directions I can take in this area of research, but I was also exposed to fascinating research opportunities not directly related to my area of expertise. My mentor and I took this research further and now it is published in the Journal of Respiratory Research. I am grateful for the opportunity that this ORCA grant afforded me to perform my research and share it with the scientific community.
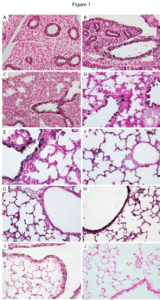
Figure 1- The temporal/spatial arrangement of α5 nAChR subunits during mouse lung development. α5 is observed as the black staining in the lung sections to the left. α5 was primarily detected in primitive respiratory epithelium at E13.5 (A) and E15.5 (B) and only minimally detected in mesenchyme. During the saccular stage of lung development (E18.5, C and PN1, D), α5 was prominently located on respiratory epithelium in the larger airways (arrows). Expression of α5 in airway epithelium was diminished at PN4 (E) and PN10 (F) and common in distal lung epithelium. At PN20, robust expression of α5 was again detected throughout the proximal lung airways (G) and expression persisted in the periphery (G) at the completion of alveologenesis. No immunoreactivity was observed in PN20 lung sections incubated without primary antibody (H). These highly regulated changes in α5 expression suggest that α5 is involved in signaling mechanisms during lung development.
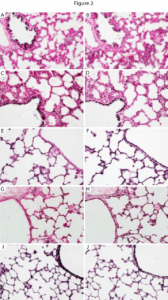
Figure 2- This is an example of the staining I performed when determining which cell types in the lung expressed the α5 protein. Staining of α5 is co-localized with other cell specific proteins in order to assess which cell types express α5 at a specific time point ( E 18.5, immediately before birth. α5 (A, C, E, G) was observed in cells that also express FoxA2 (B, D, F, H). Prominent co-expression was observed in airway epithelium at E18.5 (A, B), PN1 (C, D), and PN20 (G, H). Co-expression of α5 and FoxA2 was also detected in respiratory epithelium at PN1 (C, D), PN4 (E, F), and PN20 (G, H). These cells can now be studied further to observe the specific role that the α5 subunit plays at this time of development.
