David Barnes and Dr. Jeffery Barrow, PDBIO
Limb formation in tetra pods, animals with four legs, starts with the outgrowth of lateral plate mesoderm at the level of both forelimb and hind limb. A ridge of ectodermal tissue located at the distal margin, named the apical ectodermal ridge (AER), plays an essential role for the outgrowth and patterning of limbs. Patterning is defined as molecular or morphological differences that are established along an axis. The limb is patterned along each of its three axes: along the proximal to distal (PD) axis, the nature of the limb changes from humerus/femur (stylopod) to radius, ulna/tibia, fibula (zeugopod), to hand/foot (autopod). Anterior posterior (AP) axis patterning in the fore limb is defined as radius and thumb anteriorly and pinky and ulna posteriorly. Finally, the limb is patterned along the dorsoventral (DV) axis with the palm of the hand being ventral and the back of the hand being dorsal. The embryonic precursor to the vertebrate limb is the limb bud. The limb bud is composed of loosely organized cells that will give rise to the limb skeleton known as the limb mesenchyme. These cells are jacketed by overlying surface ectoderm which will later form the epidermis that covers the limb.
After the discovery of the AER, experiments were carried out to determine its significance. One experiment, done by J.W. Saunders, involved the complete removal of the AER in early limb development. This resulted in the formation of severely truncated limbs containing only proximal structures (i.e., stylopod). However, at later time periods, removal of the AER allowed for increasingly more distal structures [3]. This experiment indicated that the AER is essential in controlling proximal to distal limb outgrowth in a time dependent manner (i.e. proximal structures form first followed by more distal structures). The question of exactly how the AER regulates limb outgrowth and patterns the limb in a proximal to distal fashion has been the focus of extensive research for the past 60 years.
My research is part of a new model proposed by Dr. Jeffery Barrow and his laboratory: the mesenchyme recruitment model. We propose that the mesenchymal cells in the limb bud are recruited toward the AER by the action of a protein gradient. Cells will grow directionally out towards the AER instead of growing in all directions. It has been found that members of the fibroblast growth factor (FGF) family are expressed in the AER and are sufficient to restore distal outgrowth and patterning to limbs lacking the AER [1,2]. When FGF is absent in the AER, limbs do not develop and the mutant limb buds are significantly smaller than controls. Surprisingly, this is not due to decreased cell proliferation or increased cell death [4]. This suggests that FGFs must mediate outgrowth in mechanisms other than promoting mitosis or cell survival; which is consistent with our proposed model. Studies in Dr. Barrow’s lab have given insight into how the AER recruits cells. For example, FGFs from the AER induce the expression Wnt5a in gradient fashion. This gradient provides a directional cue to the mesenchymal cells, telling them to grow distally. If the AER is recruiting mesenchymal cells to grow distally toward it, it makes sense that the dimensions of the AER are critical in shaping the mesenchyme that follows.
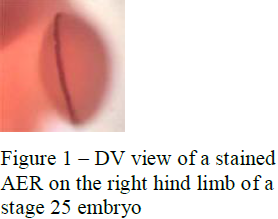
To test this hypothesis I measured the AP length and DV thickness of the AER of chicken embryos at different developmental stages and compared that data to the AP length and DV thickness of the skeletal elements (i.e., stylopod, zeugopod, autopod) of older embryos. I dissected embryos at times when the AER first appeared (around 76-hrs. or stage 18) and then at 4-hour intervals until it disappeared (around 6 days or stage 26). Hambuurger-Hamilton (HH) stages were used to determine the age of the embryos instead of hours to ensure that each embryo I used was placed in the proper age group (not all 76 hour embryos will be have developed at the same rate, HH stages are much more accurate in determining chick embryo age than hours). Five to six embryos were used for each stage. I then subjected these embryos to Fgf8 in situ analysis to mark the presence of the AER. In this process the AER was marked using a Fgf8 probe and then stained using BM purple, this allowed the AER to be visualized and measured. The in situ staining was a 4-day process. Photographs were taken to allow measuring of the AP and DV lengths of the fore limbs and hind limbs (see figure 1).
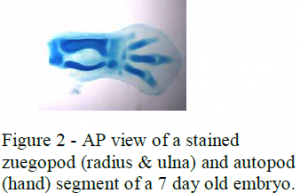
I also measured the AP length and DV thickness of the skeletal structures of the stylopod, zeugopod and autopod of older embryos (7-day embryos) to determine how these measurements correlate with the AER measurements of the younger chick embryos. The limb segments of the 7 day embryos were dyed using coomossie blue and then prepared into gelatin molds. These were then sectioned into 150 micrometer segments so the AP and DV lengths could be measured proximally to distally. Photographs were also taken of these so the AP and DV lengths could be measured (see figure 2).
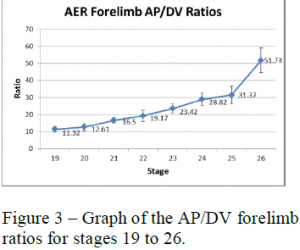
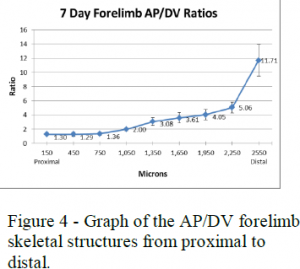
To analyze these measurements I used length to width (AP to DV) ratios of the AER and skeletal structures. The AP to DV ratios of the young embryos for each stage were averaged and then plotted against the different stages of chick development from early to late (see figure 3). The AP to DV ratios of the skeletal structures of the 7-day embryos were also averaged for each segment of the limb and plotted against the different sections of the limb from proximal (stylopod) to distal (autopod) (see figure 4).
Figures 3 and 4 show the data collected for the forelimbs of the chick embryos. These graphs show that the AER AP/DV ratio steadily increases as the age of the embryo increases with a sharp increase around stage 25. The AP/DV ratio also increases proximally to distally for the skeletal structures with a sharp increase at the end where the digits are forming. These graphs show a correlation between the AER AP/DV ratio and the AP/DV ratio of the forming skeletal structures of the embryo.
The data shows that the shape of the mesenchyme, which becomes the skeletal structures, are influenced by the shape of the AER. This research helps validate the mesenchyme recruitment model proposed by Dr. Barrow’s lab and gives researchers a better understanding of limb growth and patterning in tetra pods. Limb abnormalities are some of the most frequent birth defects, happening in about one per one thousand births [2]. This data, along with the rest of Dr. Barrow’s research, which will be published in the journal Development, will potentially be very valuable in treating and preventing limb abnormalities.
References
- Fallon, J. F., et al., 1994. FGF-2: apical ectodermal ridge growth signal for chick limb development. Science. 264, 104-7.
- Niswander, L., et al., 1993. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 75, 579-87.
- Saunders, J. W., Jr., 1948. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool. 108, 363-403.
- Sun, X., et al., 2002. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 418, 501-8.