Eric R. Griffiths and Dr. Greg Burton, Microbiology
During HIV infection, blocking viral replication has drastic effects on HIV immune complexes maintained on follicular dendritic cells (FDCs): HIV immune complexes quickly drop off FDCs to undetectable levels (1). This was a remarkable and unexpected finding since FDCs typically preserve trapped antigen for months to years. Furthermore, mice have been shown to carry infectious viral immune complexes on FDCs for periods of over nine months in the total absence of viral replication (2).
I reason the discrepancy between the contradictory findings in mice and men is that during HIV infection the virus replicates to high levels which “super saturate” the FDC antigen trapping network. Furthermore, when viral replication is blocked, the FDC network rapidly returns to more physiological levels of trapped virus (picogram amounts) on the FDC network, and these may escape detection.
I am testing my hypothesis by establishing an active retroviral infection in mice and then blocking viral infection and measuring the decline of FDC associated retrovirus. Blood samples from each mouse will be analyzed for p30, the homolog of p24 in HIV, using antigen capture ELISA. FDC viral burden will be assessed by lymph node isolation and quantifying virus present by antigen capture ELISA. The FDC-viral burden will then be correlated with the effectiveness of the drug therapy by comparing p30 values.
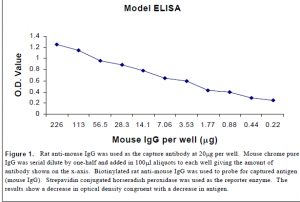
The antigen capture ELISA for p30 is not commercial available. Therefore, I worked in conjunction with another colleague in developing this assay so that it was sufficiently sensitive to detect picogram levels of p30 antigen. I first developed a working ELISA using rat anti-mouse antibodies for the capture and biotinylated rat polyclonal anti-mouse antibodies for probes. Mouse Chrome pure IgG was used as the antigen. I experimented with different concentrations of capture and probing antibodies and also with different buffers and pH. Once I established a working model I used the same protocol to develop a working p30 assay.
In conjunction with the ELISA a p30 standard was made by growing up frozen viral stocks of LP-BM5 and then lysing the virus in lysis buffer and collecting the p30 protein by centrifugation. After polyacrylimide gel electrophoresis (PAGE) of the p30 samples, it was silver stained and compared with know values of bovine serum albumin that were run using PAGE also (data not shown).
Due to the time involved in developing the ELISA, I wasn’t able to complete the experiment. The next steps, however, that I am working on are 1) reverse transcriptase (RT) assays which will test the infectivity of the LP-BM5 viral stocks that will be used to infect the mice and 2) In vitro studies of the inhibitory effects of (R)-9-(Phosphonylmethoxypropyl)adenine, (PMPA) on the viral reverse transcriptase.
In order to block viral replication, I had originally proposed using azidothymidine (AZT) and Crixivan, a protease inhibitor; however after more research in the literature we found that PMPA will effectively shut off viral replication (3). Therefore, we reasoned it would serve better in our model by substituting the PMPA for both the AZT and Crixivan.
After establishing the infectivity of the viral stocks and completing the RT assays, I will infect the mice and continue the experiment as outlined in the proposal.
References
- A. T. Haase et al., Science 276, 960 (1997).
- G. F. Burton et al., Immunol. Rev. 156, 185 (1997).
- Y. Suruga et al., AIDS and Hum Retrovirol. 184, 316 (1998).