Kimberly Jo Davis and Dr. Rex G. Cates, Botany and Range Science
There are many approaches to collecting medicinal plants. I compared results from two plant collection approaches, the ethnobotanical-based collection and ecologically-based collection. The ethnobotanical approach is based on plant use by native people. The ecological approach is based on plant life history principles i.e.; 1) plants living in stressed environments are rich in secondary metabolites, and 2) more predictable tissues and plants (i.e. evergreen perennials, mature tissues) have a diverse set of chemical defenses.1 Most medicinally active properties are from secondary chemicals.2 Plants from the Sonoran Desert typify these principles.
The plants were collected in the Tucson, Arizona area in spring, 1998. They were separated into the two study groups, ethnobotanical and ecological. Information about ethnobotanical use came from a Yaqui Indian healer, and a literature search of major periodical databases.
Fresh bark was ground to a powder in liquid nitrogen. Plant tissue was extracted in three solvents to separate chemicals of differing polarity. The hexane fraction extracted non-polar chemicals, the methanol dissolved polar organic chemicals, and water extracted polar chemicals. Additionally, the water fraction mimics the indigenous plant preparation, because often plants were prepared as infusions. Each extract was taken to dryness and stored at -80 °C in an ultralow freezer.
Plant extracts were tested on HeLa cervical cancer cells to evaluate potential anticancer activity.3 Sulfarodamine B dye assay was shown to be that most accurate method to measure cancer cell death. 3 Extracts were also tested on 3T3 fibroblast cells to see if the plant extracts were harmful to noncancerous cells.4
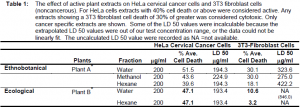
In both groups of plants, anticancer activity was not localized to a specific polarity. Results show that out of 36 total extracts, there were six active methanol extracts, five water extracts, and three active hexane extracts. This suggests that different chemicals have anticancer capabilities. Fourteen of the thirty-six extracts actively killed cancer cells at a level of 40% cell death or greater at 200 mg/ml. Out of twenty-seven extracts from the ethnobotanical group, eleven showed 40% or more cell death at 200 mg/ml.
The ecological plant group had three active extracts from the nine tested. The methanol extract from Plant A* caused 78.6 % HeLa cancer cell death. Unfortunately, it also caused about the same amount of cell death in the 3T3 fibroblast cell line. Alternatively, Plant B* showed 47.1 % activity in both the water and hexane fractions. What is most exciting is that both extracts from this plant showed very little activity on the 3T3 fibroblast cells. This suggests potential for further development and research for cancer activity. From the total tested extracts, five active extracts killed less than thirty percent of normal 3T3 cells. (Table 1).
Currently, collaboration is underway with the National Cancer Institute, Natural Products Division for further testing of Plant B*.
References
- Rhoades, David F. and Cates, Rex G. 1976. Towards a general theory of plant herbivore chemistry. Recent Advances in Phytochemistry 10: 168-213.
- Capson, T.L., P.D. Coley, T. A. Kursar. 1996. A new paradigm for drug discovery in tropical rainforests. Nature Biotechnology 14: 1200-1201.
- Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, D.V., Warren, J.T., Bokesch, H., Kenney, S., Boyd, M. R. 1990. New Colorimetric Cytotoxicity Assay for Anticancer- Drug Screening. Journal of the National Cancer Institute 82:1107-1112.
- Resources from the Natural Products Research Center supported this research.
* No plant names were included in order to protect future intellectual property and patent rights