Darin V. Allred with Kim L. O’Neill, Microbiology
Apoptosis is a term used to describe a programmed method of cellular suicide. This programmed cell death is a normal and very important feature of the maturation of an organism. The body uses it to tightly control the number of divisions of all types of cells in order to eliminate excess cells and develop properly. For example, our fingers are separated because embryonic cells that once joined them died during development.
The inability of cells to undergo apoptosis is an important cause of many disorders, including cancer. Cells unwanted by the body are normally signaled to kill themselves. However, when something goes wrong with the apoptotic mechanism, these cells can proliferate uncontrollably and become tumors. Since apoptosis is such an important protection against cancer, it has been the subject of very intense research ever since its importance was first understood in 1972 [1].
During the course of apoptosis, several key steps occur such as a loss of cell volume, DNA degradation, and alterations in the membrane. Although the nature of these membrane changes is still poorly understood, it is known that during programmed cell death, the plasma membrane becomes susceptible to attack from an enzyme called secretory Phospholipase A2 (sPLA2) [2].
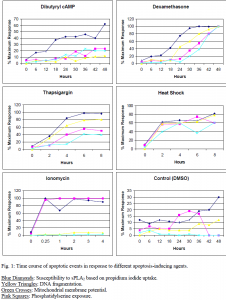
The purpose of this study was to identify the timing of this susceptibility relative to the other events that occur during apoptosis. Death was induced in S49 Lymphoma cells by five different known apoptosis-causing agents. These include dexamethasone, dibutyryl cAMP, ionomycin, thapsigargin, and heat shock (1 hour at 43° C). Each produces a different time course of apoptotic events. Control cells were treated with DMSO, as it is the solvent in which the above agents were dissolved. Sensitivity to sPLA2 was determined by propidium iodide uptake. Propidium iodide is a fluorescent dye that is unable to pass into healthy cells but can enter cells whose membranes have been hydrolyzed by sPLA2.
The appearance of membrane vulnerability to sPLA2 was compared to the following events: loss of mitochondrial membrane potential, phosphatidylserine exposure in the outer leaflet of the cell membrane, early DNA damage assessed by the comet assay, and cell shrinkage assessed by flow cytometry. It was found in each case that susceptibility to sPLA2 occurred concurrent with or earlier than each of the measured events suggesting that early membrane changes occur during apoptosis that render the cells vulnerable to the enzyme (Fig. 1). Therefore, sensitivity to the sPLA2 is not only a reflection of dead cell remnants, but probably a representation of more subtle changes to the cell membrane that arise during early stages of apoptosis.
The focus of my work on this project was to measure DNA damage with the comet assay. Kelli H. Neilson, a graduate student in Dr. John Bell’s lab in the Zoology Department, performed the remainder of the assays and was the primary scientist and author of the project. The money awarded by ORCA allowed me to travel to the conference of the American Society for Cell Biology in San Francisco in December of 1998 and assist Kelli in presenting our results before the body of the Society. A complete manuscript of the project has been submitted to Biochimica et Biophysica Acta for publication.
References
- J.F. Kerr, A.H. Wyllie, A.R. Currie, Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics, Br. J. Cancer 26 (1972) 239-257
- G. Atsumi, M. Murakami, M. Tajima, S. Shimbara, N. Hara, I. Kudo, Biochim Biophys Acta 1349, 43-54