Niloufar Tabatabaei, Department of Zoology
Introduction
Pulmonary Hypoplasia is a lethal neonatal disorder associated with the reduction of thoracic volume due to skeletal dysplasia.’ The homozygous recessive chondrodysplasia (cho/ cho) in mice serves as our model enabling us to demonstrate how the cartilage matrix is tied together on a molecular level. Atypically wide type II collagen fibrils have been observed in the cartilage of cho/ cho fetuses. This suggests that the mutation has altered the process by which collagen is assembled into fibrils in the extracellular matrix. Recently the cho mutation was mapped to chromosome 3 which is linked to a! (XI) collagen. Based upon this knowledge an immunogold technique was applied in order to define the pathogenic mechanisms for the cartilage defect in (cho/cho) mice. The immunogold technique enables us to correlate fibril diameter with the extent of distributed al(XQ antibodies in the cartilage matrix fibrils in the heterozygote (carriers for the cho mutation). My project is to conduct and experiment with an immunogold technique described by Dr. Douglas Keene and associates.’
Materials and Methods
Immunolabeling of Tissues: Eighteen day old mouse cartilage was used or the enbloc immunolabeling of tissues. Fresh tissues were obtained and trimmed 0.5-1.0 mm in thickness. The unfixed tissues were immediately immersed in PBS, pH 7.4 at 4’C for 15 min. Then the tissues were incubated in chondroitinase ABC and kept overnight at 4’C Subsequently the tissues were washed in PBS five times within a 2 hr. time frame and kept at 4’C. Next the primary antibodies were diluted with PBS I :5 and applied to the tissues, this was kept at 4’C. overnight. The antibodies that we used were Rabbit 16123 (XQ, Rabbit 4876 (XI), Rabbit (20545 (XQ, Rabbit 20545 (XI), Rabbit 9264 (IX), Goat anti-II, Rabbit anti-decorin 1909, Rabbit 1851 anti-XII, and Rabbit 4099 anti-XIV. Twenty four hours after the application of the primary antibody the tissues were washed again in PBS 3X, each wash for an hour at 4’C Next the colloidal gold conjugate Goat anti Rabbit (GAR) was applied to the tissues and kept overnight at 4’C. After the incubation period the tissues were washed in PBS at 4’C. on a rotating stage. Then they were rinsed in 0.1 M Cacodylate Buffer pH 7.4 for 15 min at 4’C, following which they were fixed in 1.5% glutaraldehyde and 1.5% paraformaldehyde in 0.1 M cacodylate pH 7.4, and tannic acid for I hr at 4’C. Finally the samples were rinsed again in buffer, dehydrated in a graded series of ethanol dilutions to 100% EtOH, infiltrated in Spurr’s resin and embedded.
Conventional and High Voltage Transmission EM: For conventional ransmission EM, 60-90nm sections were cut using a Sorvall MT-2B ultramicrotome and mounted on 3 mm formvar-coated grids. Sections were vapor stained with 2.0% Os04 then stained with 5% uranyl acetate for 15 min, washed with 50% EtOH imd distilled water and stained in Reynolds’ lead Citrate’ for 60 sec. The sections were examined using a Philips EM 400 transmission electron microscope.
Results and Discussion
The initial project was to include a comparison of two types of collagen. However, this was modified to learn and develop the techniques for future comparison studies. Standard TEM procedures were learned and practiced, following which Dr. Douglas Keene visited BYU to train interested personnel in the use of immunocytochemical procedures relative to collagen.
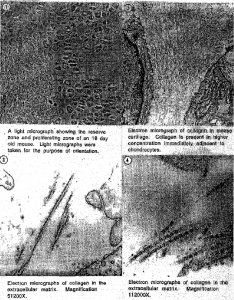
Our study of collagen was on femoral head cartilage, chondrols, and humeral cartilage. Light sections were taken for orientation purposes. The blocks were then trimmed to include only the reserve, proliferating, and hypertrophic zones of the growth plate (Fig.!). In these areas collagen is highly dense. Following the trimming ultrathin sections were taken and observed under the microscope (Fig.2, Fig.3, Fig.4). It was noted that collagen is present in higher concentrations immediately adjacent to chondrocytes (Fig. 2). In immunogold stained sections the area of interest is limited to 100 nm and thus it is difficult to locate the labeled collagen. Perhaps in future studies, immunolabeled tissues can be labeled with ruthenium red. Ruthenium red stains intensifies areas that are labeled with gold particles.
Acknowledgements
I would like to thank Dr. Seegmiller for designing the project, and Dr. Keene for teaching the immunocytochemical procedures relative to collagen. My sincere gratitude to Dr. Gardner for teaching me, mentoring me, and providing valuable knowledge and assistance. I am also grateful to the Office of Creative Research and Work for the scholarship that supported this research project.
References
- Foster, M.]., A.P. Caldwell, J, Staheli, D.H. Smith, J.S. Gardner, and R.E. Seegmiller (1993) “Pulmonary Hypoplasia associated with reduced thoracic space in mice with disproportionate micromelia (DMM).” Anat. Rec., 238:454-62.
- Keene, D.R., E. Engvall, and R.W. Glanville (1988) “Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network.” J.Cell. Bioi., 107:1995-2006.
- Reynolds, E.S. 1963. “The use of lead citrate at high pH as an electron opaque stain in electron microscopy.” I. Cell Bioi. 17:208-212.