Bryan R McRae and Dr. Earl M Woolley, Chemistry and Biochemistry
Despite the astounding advances in modern genetics research, surprisingly little is known about the thermodynamic behavior and physical properties of DNA’s molecular subunits. Our study investigated several thermodynamic properties of common nucleic acid constituents. Specifically, we calculated the apparent molar volumes (Vφ) and apparent molar heat capacities (Cp,φ) of the nucleosides adenosine, guanosine, cytidine, thymidine, and uridine, and of their corresponding bases adenine, cytosine, thymine, and uracil, in dilute aqueous solutions.
Each of the many nucleotide subunits within DNA or RNA nucleic acid polymer chains has three chemically-linked components: 1) a nucleic acid base, generally adenine (A), guanine (G), cytosine (C), thymine (T), or uracil (U); 2) a sugar (deoxyribose or ribose); and 3) a phosphate group. Nucleosides lack the phosphate, referring instead to the base + sugar. In our study, all of the nucleosides contained ribose sugars except for thymidine, which is found only in DNA and thus contained deoxyribose.
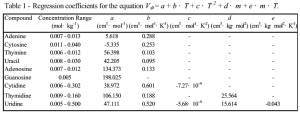
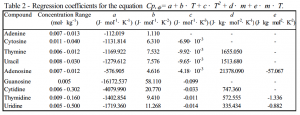
After preparing solutions of several different concentrations of each compound, we measured the solutions’ densities using an Anton Paar DMA 512 vibrating-tube densimeter at temperatures from 5º C to 95º C, at a pressure of 2.6 atmospheres. Recorded values each represented the average of 30 consecutive measurements under stable conditions at each temperature. An entire scan for one solution required about 12 hours to complete. We then measured the solutions’ specific heat capacities from 5º C to 120º C using a NanoDSC 6100 differential scanning calorimeter from Calorimetry Sciences Corp. Values at each temperature represented the average of eight consecutive scans occurring over a 24-hour period. In all, we obtained data for 39 different solutions. From the densities and specific heat capacities, we calculated the apparent molar volumes and apparent molar heat capacities of the different compounds by comparing the solution results to similar scans of water alone. We then used the SigmaPlot 3-D graphing program to fit the data to regression equations describing the apparent molar properties over the experimental ranges of temperature and concentration. Due to space constraints in this report, we have provided the regression equations and coefficients for each compound (Tables 1 and 2), rather than the entire data sets. Our completed study, along with other related work, will be submitted for publication in the Journal of Chemical Thermodynamics.
Two major unforeseen obstacles arose during the course of our study. Equipment failures and instrument troubles repeatedly delayed the scans for several of our compounds, ultimately requiring some compounds to be moved to another instrument and completely restarted. Of greater consequence were our difficulties in determining the concentration ranges in which the different compounds are soluble in water. The nucleosides’ solubility decreases rapidly at colder temperatures, and little information was available for determining the compounds’ solubility limits. Furthermore, the nucleic acid base and nucleoside crystals were extremely slow to dissolve, often requiring a week or more to move into or out of solution. Initially, we chose aqueous concentration ranges similar to those of other published work,1 but we discovered that many of the more concentrated solutions, which we had heated to speed dissolving, eventually formed crystals, suggesting that the published studies had reported data from supersaturated conditions. We needed to avoid running supersaturated solutions wherever possible, to avoid potentially depositing solid crystals inside the equipment tubes. We determined the aqueous solubility limit for each compound by heating and dissolving incrementally increasing amounts of the solid crystals in water, then storing the solutions under refrigeration for a period of time to ensure they remained in solution. This slow process considerably delayed our study, but provided useful solubility data for future research. The solubility limits are reported in Table 3.
In our future work, we will use the results of this study to determine the same thermodynamic properties for the common nucleic acid bases and nucleosides in acidic and basic pH-buffered solutions.
References
- Kishore, N.; Bhat, R.; Ahluwalia, J. C. Biophys. Chem. 33, 227-236 (1989).