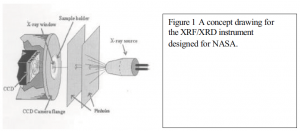
Brent Kimball and Dr. Larry V Knight, Physics
My proposition was to determine the feasibility of using a combination x-ray fluorescence/x-ray diffraction technology (initially designed to identify strictly inorganic compounds) for the analysis of organic molecules. By modifying a mineralogical device created for NASA it is feasible to identify solid organic compounds such as drugs, harmless compounds like glucose, and some inorganic compounds that look like drugs. Emergency teams, Forensic Officers, and Police Departments and even drug manufacturers will benefit from the applications. I will provide a brief background on diffraction, clearly describe my findings, and provide proposals to further develop a working technology.
X-rays are electromagnetic radiation of wavelength about 1 Å, about the same size as an atom. X-ray diffraction has been in use in two main areas, for the fingerprint characterization of crystalline materials and the determination of their structure. Each crystalline solid has its unique characteristic X-ray powder pattern which may be used as a “fingerprint” for its identification. Once the elemental composition has been identified through fluorescence data collection, X-ray crystallography may be used to further determine its structure, i.e. how the atoms pack together in the crystalline state and what the inter-atomic distance and angle are etc. X-ray diffraction is one of the most important characterization tools for unknown compounds. It can determine the size and the shape of the unit cell for any compound most easily using the diffraction of x-rays. For this reason the bulk of my research is focused on diffraction rather than fluorescence.
For my research I used some diffraction data collected from the XRF/XRD device developed under NASA.
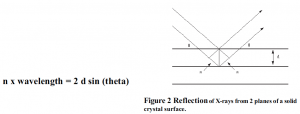
The primary conflict involved with identifying organic substance diffraction patterns is resolution and effective step size. The mineralogical instrument has a maximum resolution of .4 degrees. I believe that changes must be made to increase resolution. Because organic compounds are more complex and more largely spaced than their inorganic counterparts, it is necessary to have a resolution of less than .1 degrees full-width half-max 2 theta (FWHM) in order to resolve diffraction peaks. Bragg’s diffraction law describes how increased wavelength of the x-ray source will increase the angle between characteristic diffraction peaks:
Bragg’s law describes how increased wavelength of the x-ray source will increase the angle between characteristic diffraction peaks. Thus, I recommend altering the x-ray source from the Copper tube to another source characteristic of a higher wavelength x-ray such as Chromium (As suggested by my associate Sterling Cornaby, Moxtek Engineer).
Using the BYU diffract-o-meter, I collected data for a few simple organic compounds. This helped me to determine the step-size increase necessary to accurately identify organic molecules. By adjusting the step-size I found that organic molecules require more precision than inorganic molecules. The step size I began with was approximately .01 degrees. Through my research I found that .05 degrees of resolution is sufficient to resolve the peaks of the proposed compounds. This can be done by moving the sample further from the CCD. By doing this, the range of collectable data is decreased from approximately 50 degrees 2 theta to approximately 25 degrees 2 theta. As a result I must also recommend increasing the size of the CCD.