Katie L Jensen and Dr. Paul B Savage, Chemistry and Biochemistry
Pathogenic bacterial resistance to antibiotics has risen sharply over the past two decades.1 This problem is compounded with Gram-negative bacteria, which, in addition to developing specific resistant mechanisms, also display membrane structures that are impermeable to many hydrophobic antibiotics. Thus, new antibiotics are needed that do not elicit the emergence of resistant organisms, especially against Gram-negative bacteria.
Cationic steroid-derived antibiotics2 (CSAs) display a broad range of antibacterial properties, including activities against multi-drug resistant bacteria.3,4 Examples of these antibiotics display two types of activity against Gram-negative bacteria, one rapidly lethal to these organisms and the other sub-lethal sensitization of the bacteria to hydrophobic antibiotics.5 The purpose of this project is to investigate causes of the two different activities by analyzing where they localize in bacterial cells via electron microscopy, thus giving insight into the antibiotic’s mechanism.
The steroid antibiotics alone cannot be seen using conventional microscopy techniques. We functionalized the antibiotic with thiol groups to allow the attachment of gold nanoparticles to the CSAs, which now can be viewed using electron microscopy, allowing us to see where they localize in the bacteria.
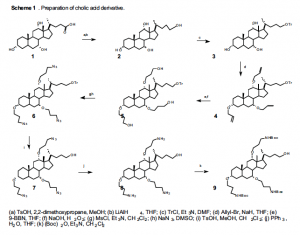
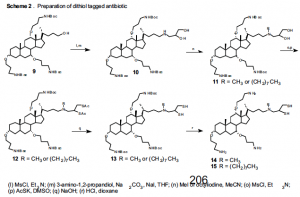
Preparation of the dithiol-tagged antibiotics with a secondary amine has been accomplished as outlined in the following schemes, without the alkylation. The cholic acid is first derivatized with protected amines and a free alcohol as shown in Scheme 1. Addition of 3-amino-1,2- propanediol and the eight-carbon hydrophobic chain will give us compound 11. Substitution with thioacetate and deprotection gives the desired antibiotic 15 (see Scheme 2) for electron microscopy studies. Similarly, antibiotic 14 will be synthesized with a methyl group, as outlined in Scheme 2.
The minimum inhibition concentrations (MICs) of the CSA lacking a hydrophobic chain was determined, and now we will treat cultures of Gram-negative bacteria this compound. After fixing and sectioning the treated bacteria, we will treat them with a suspension of gold nanoparticles. Because sulfur binds strongly to gold, the dithiol groups on the antibiotics will bind the gold nanoparticles. We will then observe the gold nanoparticles via electron microscopy. The location of the gold will indicate the location of the antibiotics within the cell.
Comparing the gold localization in the bacteria with each antibiotic will indicate the differences among the CSAs with the different types of activity. This information may also suggest the differences in the mechanisms of the lethal and the sub-lethal antibiotics. We expect the CSA containing a long-chained hydrocarbon, 15, to traverse the outer membrane of Gram-negative bacteria by self-promoted transport and be found in both the outer and inner membranes.
However, the antibiotic with only a methyl group, 14, should remain in the outer membrane. The activities of these CSAs are expected to parallel their locations within the cell. We anticipate 15 to be the lethal antibiotic, while 14 should sensitize the bacteria.
These studies will allow us to determine the causes of the two different activities associated with our antibiotics. From their locations in the bacteria, we can gain insight into the antibiotic’s mechanism. Also, CSAs likely share a mechanism with cationic peptide antibiotics. Due to their large size, cationic peptide antibiotics are difficult to purify and derivatize.6,7 Our study will provide insight into the mechanism of CSAs and possibly into the mechanism of action of many peptide antibiotics.
References
- Hileman. B. Furor. Chem. Eng. News 2001, 79, 47-48.
- Guan, Qunying; et al. Org. Lett. 2000, 2, 2837-2840.
- Li, Chunhong; et al. Antimicrob. Agents Chemother. 1999, 43, 1347-1349.
- Schmidt, E.J.; et al. J. Antimicrob. Chemother. 2001, 47, 671-674.
- Li, Chunhong; et al. J. Am. Chem. Soc. 1999, 121, 931-940.
- Hancock, Robert E.W.; Chappel, Daniels. Antimicrob. Agents Chemother. 1999, 43, 1317- 1323.
- Lehrer, Robert I.; Ganz, Tomas. Curr. Opin. Immu. 1999, 11, 23-27.