Sarah Hart and Dr. Barry Willardson, Chemistry and Biochemistry
Background
Eukaryotic cells employ G protein coupled receptors (GPCRs) to detect various extracellular signaling molecules, including many that control cell proliferation. As a result, a knowledge of G protein pathway components and their mechanisms of interaction and regulation is essential in understanding cell transformation. Phosducin-like protein (PhLP) is a broadly expressed regulator of G protein signaling that exerts its effect by binding to G protein âã subunits [1-3]. When a signaling molecule binds to the extracellular surface of the receptor, it results in a conformational change in the receptor. This causes an interaction between the receptor and a heterotrimeric G protein on the intracellular surface of the membrane, catalyzing the exchange of GDP for GTP on the Gá subunit. In its GTP-bound conformation, Gá dissociates from the Gâã subunit complex and the receptor. Both GáGTP and Gâã are then free to interact with effector enzymes or ion channels and thereby regulate their activity. These effectors in turn control second messenger concentrations and kinase cascades that dictate intermediary metabolism, cell growth and differentiation [4-5].
Our lab has shown an interaction of PhLP with eukaryotic cytosolic chaperonin. Many proteins require interaction with chaperones to attain their native conformation. Eukaryotic cytosolic chaperonin contains eight distinct but related subunits. The T-complex polypeptide (TCP1) is one of these subunits and the complex [6] is therefore generally referred to as chaperonin containing TCP1 (CCT) or TCP1 ring complex (TriC) or cytoplasmic chaperonin, owing to its subcellular localization. CCT assists in the folding of actin, tubulin and several other protein substrates. De novo protein folding is now believed to couple to the chaperone machinery since a large fraction of newly translated polypeptides associate transiently with Hsc 70 and CCT [7]. The expression of CCT is increased in rapidly growing cells to meet the increased demands for more protein synthesis in such cells. The interaction of CCT with PhLP indicates that CCT activity could be linked to G protein coupled receptor pathways through PhLP. The purpose of these experiments was to test that hypothesis.
Results
Previous experiments in our lab have shown that CCT co-immunoprecipitates with myc epitopetagged PhLP fusion protein. Previous experiments have also shown that PhLP inhibits the folding of firefly luciferase, a native substrate of CCT. The addition of Gâã relieves that inhibition. We decided to perform an additional experiment to see if PhLP would compete with transducin, another known substrate of CCT.
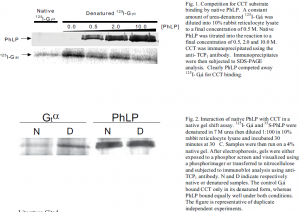
Competition for CCT substrate binding by native PhLP. To find out if PhLP would compete with a known substrate, transducin (Gtá), for binding to CCT, we incubated a constant amount of denatured 125I- Gtá with rabbit reticulocyte lysate containing CCT while native PhLP was titrated into the reaction. CCT was immunoprecipitated using the anti-TCP1 antibody and immunoprecipitants were subjected to SDS-PAGE analysis. The autioradiogram of the gel is shown in figure 1.
Interaction of native PhLP with CCT in a native gel shift assay. To address the possibility that PhLP could simply be a substrate for CCT, we performed an assay comparing PhLP binding with binding of the known substrate, transducin, in a native gel mobility shift assay. Radiolabeled 125I- Gtá, native or denatured in 7 M urea, was diluted into rabbit reticulocyte lysate. Radiolabeled 35S-PhLP was subjected to the same conditions. Samples were electrophoresed on 4% native gels and are shown in figure 2.
Literature Cited
- Miles, M.F., S. Barhite, M. Sganga, and M. Elliott, Phosducin-like protein: an ethanol-responsive potential modulator of guanine nucleotide-binding protein function. Proc Natl Acad Sci USA, 1993. 90(22): p. 10831-5.
- Thibault, D., M. W. Sganga, and M. F. Miles, Interaction of phosducin-like protein with G protein getagamma subunits. J Biol Chem, 1997. 272(19): p. 12253-6.
- Thulin, C. D., K. Howes, C. D. Driscoll, J. R. Savage, T. A. Rand, W. Baehr, and B. M. Willardson, The immunolocalization and divergant roles of phosducin and phosducin-like protein in the retina. Mol Vis, 1999. 5: p. 40.
- Gutkind, J. S., The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem, 1998. 273(4): p. 1839-42.
- Hamm, H. E., The many faces of G protein signaling. J Biol Chem, 1998. 273(2): p. 669-72.
- Lewis, V.A., G.M. Hynes, D. Zheng, H. Saibil, and K. Willison, T-complex polypeptide-1 is a subunit of a heterotrimeric particle in the eukaryotic cytosol. Nature, 1992. 358(6383): p. 249-52.
- Thulasiraman, V., C.F. Yang, and J. Frydman, In vivo newly translated polypeptides are sequestered in a protected folding environment. Embo J, 1999. 18(1): p. 85-95.