Kari Cowdell and Dr. Steven A. Fleming, Chemistry and Biochemistry
Solar energy is a major reason for the study of photochemistry. Photochemistry is the investigation of the interaction of organic compounds with light. The ability of plants and bacteria to harness the sun’s energy is an example of photochemistry. One area of photochemical research is the synthesis of cyclobutane rings which are found in plant essential oils and animal pheromones. Anticancer drugs such as taxol also have four member rings in their structures. Biological systems are unique in their ability to produce cyclobutane systems with specific stereochemistry that current methods of synthesis require great effort to acquire. It has been reported that molecules with extended ð- and aromatic systems undergo inter-system mixing through space which results in a weak attraction between bonds. This ð-ð stacking is a significant factor in DNA stability and may be a significant contributor in other biological stereoselectivity. During the past number of years, ð-ð stacking has been extensively studied in both gas- and solid state chemistry yet, for the most part, it has been ignored in the liquid phase. It is difficult to determine if ð-stacking groups align themselves in liquids. This study is designed to establish whether such interactions occur in solution. In addition, research in controlling carbon-carbon bond formation can be useful in synthetic methodology which can be useful in the synthesis of natural products or biologically active compounds.
Our group has studied photocycloadditions and has found that phenyl groups have aligned in ðstacking conformations.1 Semi-empirical molecular model calculations have shown that when phenyl groups are calculated at a distance of less that 5Å they are sufficiently close for ð -ð mixing to occur.2 This study will address whether polarized ð-systems play a role in ð-ð interactions.
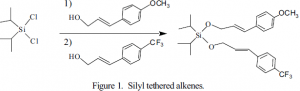
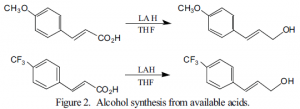
The synthesis of the compound we intend to study is shown below (Figure 1). The alcohols necessary for this synthesis, one containing an electron donating and one with an electron withdrawing group, were not commercially available. However, the corresponding carboxylic acids are available. To be covalently bonded to the silyl group, the acids must be reduced to the alcohol. That synthesis is shown in Figure 2. The yield for the p-methoxycin-namic acid reduction after purification is 38%. Reduction of the p-trifluoromethylcinnamic acid has not been optimized at this time because it is more problematic, but should be 20-40%. Both reductions are complicated by over-reduction of the cinnamic acid to give the corresponding 3- aryl-1-propanols. We have optimized the reduction of the p-methoxy compound by monitoring the reaction as the following conditions were varied: number of equivalents of LAH, temperature during LAH addition, rate of LAH addition, and time of reaction.
Irradiation of the silyl tethered compound (Figure 1) has potential for producing several different cyclobutane isomers. Time studies will determine the rate of the [2+2] addition. Solvent studies are crucial in this experiment as little is known about solvent effects in stereoselective ð stacking.3 It is known that more polar solvents, such as methanol, reduce the ability of ð-ð systems to mix. Non-polar solvents, such as cyclohexane and methylene chloride, seem to facilitate ð-stacking structures and will be examined. It is expected that small variations in solvent polarity will greatly determine the extent that ð-ð mixing occurs. These studies will allow a more accurate picture of ð-ð stacking strengths as well as what environmental controls are necessary for ð-mixing to occur.
This project will provide further information on ð-stacking. The experiment will be publishable regardless of the outcome. If ð-stacking is implicated, then it will add considerable support to the current explanation of stereoselectivity in the 2+2 photocycloaddition reaction. If the results are inconsistent with ð-stacking, then an alternative explanation for the observed selectivity will be proposed. As previously stated, these ð-ð interactions are only beginning to be understood and they may, or may not, play a significant role in the natural world. This molecule, while not having any synthetic advantage in itself, presents a easily studied model that can provide information on a wide range of details.
References
- Fleming, S. A.; Ward, S. C. Tetrahedron Lett. 1992, 33, 1013.
- Cornil, J.; dos Santos, D. A.; Crispin, Z.; Silbey, R.; Bredas, J. L. J. Am. Chem. Soc. 1998, 120, 1289.
- Shetty, A. S.; Zhang, J.; Moore, J. S. J. Am. Chem. Soc. 1996, 118, 1019.