Melissa Draper and Dr. Adam Woolley, Chemistry
Miniaturization offers several advantages in regards to protein characterization, including the potential to increase speed and throughput while reducing reagent consumption (and therefore reducing cost) during analysis. In recent years, the Human Genome Project has generated an unprecedented amount of genetic information; it has also instigated several developments in the area of miniaturization. However, one of the greatest advantages of the technique—the possibility of forming integrated microdevices capable of carrying out multiple steps of an assay on a single miniaturized platform—remains relatively undeveloped.
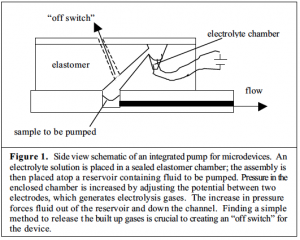
Over the past several months I have worked with Dr. Adam Woolley to develop a small elastomer device that relies on water hydrolysis to generate pressure which in turn forces a sample from one end of a channel to the other. (Fig. 1) While many possible pressure-driven pumping methods are available, electrically-actuated pumping is particularly promising because of its ability to be easily miniaturized and because it does not require additional expensive or unwieldy units, such as gas cylinders. Devices utilizing this technology may prove especially important in clinical applications, enabling the protein profiles of normal and cancerous tissues to be distinguished at an early stage without bulky or costly equipment. These devices may also eventually be employed studying the contents of singles cells, nucleic acid or carbohydrate analysis, and monitoring pollutants in the environment.
Pump control has been my primary focus at this stage in the project. Last fall I wrote a program in LabVIEW capable of initiating electrolysis by generating a voltage difference between the two leads in the electrolyte chamber. The program permitted voltage and time to be varied; it also recorded information about these variables along with resistance and current for each trial. While this set up made it easy to begin pumping, it did not include an “off” button more elaborate than the user manually breaking the seal above the sample chamber and releasing the system to atmospheric pressure.
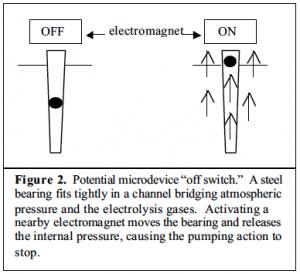
This past semester, Dr. Woolley and I have investigated several possible cessation solutions, most utilizing electromagnets. Because they are available in a range of sizes, are relatively inexpensive, and, most importantly, generate a magnetic field only when current is supplied—something easily accomplished with our pre-existing LabVIEW program—electromagnets were an obvious choice. How to best utilize the magnets was less obvious. Originally we hoped to manipulate the location of a small steel bearing in a narrow elastomer passage: when a magnetic field was induced the bearing would move towards the magnet, unblocking the electrolyte chamber, equalizing the pressure, and stopping pumping action. (Fig. 2) Unfortunately, to successfully seal initially the bearing had to be wedged in the elastomer too tightly to reliably release with the low-powered electromagnets suitable to the size of the device. Varying the diameter of the bearing (1mm to 80mm), as well as the proximity to the electromagnet did not help. Replacing the elastomer channel with a tapered plastic sleeve (such as a trimmed plastic pipet tip) was also initially encouraging, but encountered problems with adequately sealing the chamber at all times when the pump was in use. Smaller bearings with less circumference lodged against the plastic sleeve tended to erupt from their position as pressure from the electrolysis built beneath them, prematurely stopping the pump. However, a larger bearing used in this design, with the advantages in both weight and surface area, was our first successful model which could both be turned “on” and “off” on demand. The tendancy of the plastic to deform and no longer fit snug against the bearing or to alternately pull away from the elastomer used to hold it in place forced us to continue exploring other alternatives.
Abandoning the bearings, but not the electromagnets, we began looking at more mechanical options, including what at the end of the semester was our most promising approach yet: a spring-loaded release. By affixing a thin piece of elastic material to a small piece of steel suspended above an electromagnet, when the electromagnet is “on,” as controlled by the LabVIEW program, the elastic can be stretched to bring the two pieces in contact (this still has to be done by hand, unfortunately) where they will hold their position until the magnet is turned “off.” When the magnetic field disappears, the tension in the elastic rapidly forces the metal piece back to its equilibrium position. If something used to seal the electrolyte chamber is attached to the small metal piece, this snapping action can be harnessed to stop pumping at the desired moment. Glass slides and thin pieces of Plexiglas—materials previously used to take advantage of elastomer’s ability to reversibly seal to planar substrates in order to adhere the chamber and channels holding sample to the electrolyte pump itself—proved too fragile for this technique. Instead, a small plastic centrifuge tube with a small hole cut in the bottom and its tight-fitting cap attached to a piece of steel and fitted into the device how the plastic sleeves were earlier proved successful.
We have made significant progress towards a fully-functional miniaturized pump for protein characterization, and anticipate further improving the design. Future work will include refining the “off switch” to make it gentler and better-integrated, as well as further attempts to mathematically approximate the system in order to determine efficiency and optimal settings for voltage and other variables. OFF electromagnet ON