Kyrsten Ann Crawford and Dr. Gerald D Watt, Chemistry and Biochemistry
Although the earth’s atmosphere is approximately 80% nitrogen (N2) gas, this form of nitrogen is inert and must be reduced and “fixed” into a useable form of nitrogen through the process of nitrogen fixation. Nitrogen fixation is an important biological process in the earth’s nitrogen cycle because it provides nutrient nitrogen for plants and animals. The enzyme responsible for conducting nitrogen fixation is nitrogenase, which is found only in certain diazotrophic microorganisms. Nitrogenase is also capable of reducing protons (H+) and small substrates that have a terminal triple bond, such as acetylene (C2H2). One of the stated goals of this research is to investigate the substrate reducing behavior of nitrogenase to better understand the mechanism of nitrogenase catalysis. It is important to understand how nitrogenase functions so that it can be utilized to enhance agricultural productivity and also to safeguard the ecological systems.
Nitrogenase reactivity is described in two broad categories: electron activation and substrate reduction. Electron activation refers to the reduction of electrons by the Fe protein, which electrons are then transferred to the FeMoco of the MoFe protein. The substrate reduction step involves substrate interaction at the FeMoco and activation by reduction from the “activated” electrons at that site. The focus of this research is the second category: substrate reduction.
The experiments that were performed to better understand protein-substrate interaction and substrate reduction compare the enzymatic activity when the natural substrate, N2, and the artificial but commonly used substrate, acetylene, are used as substrates. The experiments also examine the effect of the component protein ratio and concentration on nitrogenase activity under these different substrates.
The results below were obtained by measuring the rate of substrate reduction against increasing substrate concentration and fitting this data to the Michaelis-Menten model. From the fits of this model, values of Km and Vmax for substrate reduction were identified. On the basis of the M-M model, we sought to evaluate both the substrate reduction and the enzyme-substrate complex formation behavior of nitrogenase catalysis by using acetylene and nitrogen as substrates under conditions where both the protein component ratio and concentration were varied.
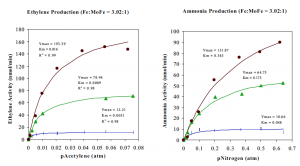
The figures below show results fromthe variation of the substrate concentration when the Fe component protein concentration is three fold greater than the MoFe component protein concentration. Acetylene and nitrogen were each examined as substrates and the rate of product formation was analyzed at this protein ratio, but at varying protein concentrations: (•) 3.43 μM Fe, ( ) 1.37 μM Fe, and ( ) μM Fe. The production of ethylene or ammonia in nanomoles per minute as a function of substrate concentration is fit to the M-M model. The fit in the following figures is shown by a smooth curve drawn through the experimentally determined data points. R2 values are measurements of the individual deviations of data points from the best-fit curves.
Our initial hypothesis was that the M-M model would apply to the substrate reduction experiments conducted by nitrogenase. We expected to see a variation in Km values with variation of substrate type, which would indicate the relative M-M constants of the acetylene and nitrogen substrates for nitrogenase. As expected, results from these figures showed that at a given protein concentration, the Km for acetylene is 0.02–0.065 times that of nitrogen. The Vmax values were greater when acetylene was the substrate than when nitrogen acted as the substrate because acetylene requires fewer electrons for reduction than nitrogen requires.
However, further examination of these reactions showed that the Km varied with protein concentration. This was unexpected behavior because Km is a fundamental characteristic of the catalytic reaction and should be constant with variation in protein concentration. Not only did the Km vary with concentration, but for acetylene and nitrogen substrates, the Km values decreased with decreasing protein concentration. These results indicate that although the activity of the nitrogenase enzyme can be fit to the M-M model at a given protein concentration, the actual nitrogenase kinetics are more complicated.
Not only were these trends in Vmax and Km values similar across substrates at an Fe:MoFe ratio of 3.02:1, but also occurred when the opposite protein ratio was used (MoFe:Fe is 2.98:1). At each of these ratios, the protein concentration was varied. The results show that the two substrates produced similar results and trends at the two ratios and as the protein concentration varied. These results indicate that there is a concentration dependence on protein activity. The concentration of the protein affects its ability to bind the substrate. However, the results also suggest that protein ratio does not influence the observed concentration dependence effect.
These results reveal that a more complex reactivity of the nitrogenase proteins for substrate reduction must occur. Such reactivity is beyond that described by the M-M model. Other work is currently being conducted for further insight into the complicated mechanism of nitrogenase reactivity.