Benjamin R. Jordan and Dr. Eric H. Christiansen, Geology
Blue Lake is located in a remote area of Western Utah, about 25 km south of Wendover, Utah. The lake has a year-round temperature of 23 degrees Celsius. This is due to the lake being fed by warm springs located at a depth of 15 m within the lake. The bottom of the lake is covered with a fine carbonate mud. The multiple inflows, however, are filled with fine sand, predominately quartz, much of which is kept in constant motion by the inflowing groundwater. Contained within this sand, and located directly within the inflows, sometimes actually suspended in them, are small, irregularly shaped nodules of calcium carbonate. These nodules are white, and contain some grains of sand within their matrix.
It was originally hypothesized that these carbonate nodules formed due to a high pH difference between the inflowing groundwater and the standing water of the lake. It was thought that as the groundwater entered the lake a calcium carbonate precipitate would form. Additionally, it was thought that precipitation was enhanced by CO2 being lost through evaporation of the lake waters (1). The irregular shapes of the precipitate were caused by the precipitate being directly in the turbulent inflows. Constant motion caused by the inflowing water, combined with variable dissolution and precipitation, produced twisted and distorted shapes.
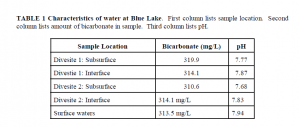
Water samples were taken of the inflowing groundwater below the surface of the lake bottom and at the inflow/standing water interface. Subsurface water was sampled by scuba divers who inserted tubing into the inflows. Containers were then placed over the end of the tubing to sample the subsurface water as it came out of the tube and before it could contact the standing water. Interface water was sampled by the divers filling containers within 20 cm of the sand bottom. Two separate sites were sampled. The surface water of the lake was also sampled. The divers also collected samples were collected of the carbonate mud and sand located at the inflows. They also collected samples of the calcium carbonate nodules.
Water samples were titrated using 1:500 HCl in order to determine the amount of bicarbonate in each water sample (Table 1).
The small differences in pH and bicarbonate levels of the inflow and standing waters refutes the original hypothesis. There is not a great enough difference in pH to immediately produce the precipitate. The waters of the lake are well mixed. In addition, a large outflow of the lake waters was found, making it unlikely that the lake has a high amount of evaporation and thus a high loss of CO2. Also, near divesite 2 an outcrop of the calcium carbonate material, similar to that found in the inflows, was found projecting up out of the lake sediment. Although near an inflow, this outcrop was unrelated to the inflow itself. Thus, it appears that the calcium carbonate is not =produced= within the inflows.
In summary, the subsurface waters are, for the purposes of this study, identical to the standing water of the lake. Complete mixing of the waters seems to have already taken place by the time the groundwater enters the lake. It is possible that the inflowing groundwater is simply recycled lake water. The lake does not appear to have a high evaporation rate. Calcium carbonate material, similar to the nodules, was found at a location unrelated to the inflowing waters.
There is some other mechanism for the formation of the nodules. Two additional hypotheses are that first, the water of the lake, as well as the groundwater, is so super-saturated with with CaCO3 that it precipitates without a great difference in pH. Although high evaporation doesn’t occur, slight losses of CO2 through light evaporation could still have a great effect on carbonate precipitation. Second, it is possible that the material that makes up the carbonate nodules existed previous to the formation of the lake. This may be indicated by the carbonate outcrop. Inflowing water then erodes and weathers this material until small pieces get caught in the inflows where abrasion of sand and dissolution/reprecipation of the calcium carbonate produce the nodules.
Future work includes comparing the chemical makeup of the outcrop material with that of the nodules as well as the chemical composition of the sand and bottom mud.