Christopher M. Runyan and Dr. William G. Pitt, Chemical Engineering
Introduction
Our lab has investigated the use of low-frequency ultrasound as an adjuvant to antibacterial therapy. We have found that ultrasound can increase killing of certain combinations of antibiotics with bacteria. Ultrasound at very low frequencies, alone, may kill bacteria; however, these frequencies are harmful to other living tissues and can not be used in a clinical setting. While ultrasound does not kill bacteria at slightly higher frequencies, it can enhance antibiotic killing, a phenomenon known as the “bioacoustic effect.”
The mechanism of the bioacoustic effect has yet to be determined conclusively. Ultrasound causes cavitation in liquids; wherein microbubbles oscillate in size causing shear forces that may damage cells. We hypothesized that one mechanism for the bioacoustic effect was that ultrasound temporarily increases the outer membrane (OM) permeability of bacteria to antibiotics, and that this enhanced permeability is due to cavitationally-induced OM perturbation.
To test this hypothesis I used the lysozyme-lysis and the nitrocefin-hydrolysis assays with the gram-negative bacteria, Escherichia coli and Pseudomonas aeruginosa. Lysozyme is an enzyme that weakens cell walls but is limited in its action in that it is too large to pass through an intact OM. An ultrasonic enhancement of passage of lysozyme across the bacterial OM would result in cell lysis measurable spectrophotometrically. Nitrocefin is a chromogenic β-lactam antibiotic that gives a color change in the presence of β-lactamase. With our bacteria, β-lactamase is located in the periplasmic space, and any spectrophotometric color change is indicative of the passage of the β-lactam through the OM. The first assay was used with both strains of bacteria and the second was used only with P. aeruginosa, as E. coli was not naturally resistant to the passage of nitrocefin into the periplasmic space. Here I report that ultrasound enhances permeability of the OM of E. coli and P. aeruginosa to lysozyme and nitrocefin, respectively. This enhancement is likely a contributing mechanism of the bioacoustic effect.
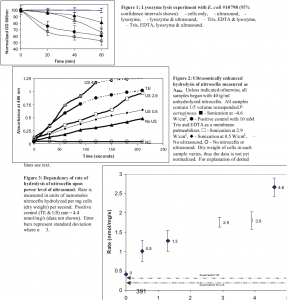
Results and Discussion
With E. coli, the presence of 1 mg/ml lysozyme produced 20% cell lysis in 1 hour. The same culture additionally exposed to 70 kHz ultrasound (800 mW/cm2) showed 35% lysis (see figure 1). Sonicating an E. coli culture that had been exposed to lysozyme for 45 minutes did not result in a sharp decrease in cell lysis (data not shown). This suggests that the lysis seen was due to a sonically enhanced OM permeabilization to lysozyme and not to the lysing of lysozyme-weakened cells by ultrasound. This enhanced cell lysis is significant (p=0.032), and noteworthy because the size of lysozyme (13,500 Da) makes it virtually impossible to pass through the porins of E. coli, which typically allow passage of small solutes < 600 Daltons. Ultrasonic enhancement of lysozyme-caused lysis was confirmed by standard plate counts (data not shown). The P. aeruginosa cultures showed no increase in cell lysis when cells in lysozyme were exposed to ultrasound. Cultures not exposed to lysozyme experienced no cell lysis.
The nitrocefin outer membrane permeability assay was used only with P. aeruginosa. Figure 2 contains a sample of the data collected before normalizing for dry cell weight. Because an increase in absorbance at 486 nm is indicative of an increase in nitrocefoic acid, the slopes of the lines in figure 2 are related to the rate of passage of nitrocefin through the OM. For most of the data points, the slope or rate remained constant throughout the experiment. However, the hydrolysis for the positive control and 4.6 W/cm2 samples did not maintain a constant rate for the entire three minutes. By adding additional nitrocefin to these samples when the rates decreased I found that this phenomenon was due to a depletion of the substrate. Thus, only the data in the linear region (solid lines) was used to calculate a rate of hydrolysis. An average hydrolysis rate was calculated from a linear regression of the linear portion of the data in figure 2. These rates were normalized for dry cell weights in the different experiments.
Figure 3 shows the dependency of the rate of nitrocefin hydrolysis on the power density of ultrasound. There is a basal rate of hydrolysis, even without ultrasound, that I posit is due to a minor flux of nitrocefin through the OM and due to the activity of â-lactamase that has been freed from damaged cells. Indeed, the rate of hydrolysis occurring in the supernatant that was cleared of cells is only slightly lower than the basal rate in the presence of cells (see dotted lines in figure 3). As can be seen, there is a correlation between of the rate of uptake of nitrocefin into the cells and the intensity of ultrasound. The permeability of the outer membrane towards nitrocefin increases as ultrasonic power density increases.
We have previously hypothesized that high local fluid velocities due to ultrasonically-induced oscillating cavitation bubbles may lead to transient perturbation of the outer membrane (1, 2). The observation that ultrasound can increase the entry of lysozyme into E. coli and of nitrocefin into P. aeruginosa supports this hypothesis. These results with large hydrophilic molecules (nitrocefin and lysozyme) complement previous work with a small hydrophobic molecule, stearic acid, whose transmembrane transport was enhanced by ultrasound (2). That work could not establish whether the enhanced transport was through the lipid bilayer or through holes in the lipid bilayer. The current work strengthens our hypothesis that ultrasound creates holes or perturbations in the lipid bilayer sufficiently large for larger water-soluble molecules to pass through.
As we deepen our understanding of the mechanisms of the bioacoustic effect we are more qualified to and certainly hope to be able to predict and demonstrate clinically relevant uses of ultrasonic adjuvant therapy. The understanding that low-levels of ultrasound can increase outer membrane permeability is likely part of the mechanism contributing to the bioacoustic effect. How ultrasound increases outer membrane permeability remains an important question. Because the passage of lysozyme is presumably through the phospholipids in E. coli it seems that a mechanism of ultrasound is a perturbation of this bilayer. Results from the nitrocefin experiments neither support nor oppose this hypothesis. Because of its small size, the sonically enhanced passage of nitrocefin could have occurred through porins or through the bilayer. Similar experiments with porin-deficient mutants would help answer this question. If the mechanism of increased OM permeability due to high sonic shear forces is related to a perturbed lipid bilayer, it would be interesting to ascertain the involvement of perturbed LPS as a contributing factor. Similar experiments with deep-rough mutants, which lack LPS, may help to address this question. Additional experiments involving a broader range of probes, both hydrophilic and lipophilic, with a varied range of sizes, could further help us define this mechanism at a molecular level.
Works Cited
- Qian, Z., R.D. Sagers, and W. G. Pitt. 1998. Investigation of the mechanism of the bioacoustic effect. J. Biomed. Mater. Res. 44:198-205.
- Rapoport, N., A. I. Smirnov, A. Timoshin, A. M. Pratt, and W. G. Pitt. 1997. Factors affecting the permeability of Pseudomonas aeruginosa cell walls toward lipophilic compounds: effects of ultrasound and cell age. Arch. Biochem. Biophys. 344:114-124.