Ethan A. Mastny and Dr. Calvin H. Bartholomew, Chemical Engineering
The objective was to develop an efficient procedure for depositing silica washcoats onto cellular, ceramic monoliths while ensuring that the silica maintains its desired properties of surface area and pore structure and is stable against erosion. Catalyst coated ceramic monoliths find application in automotive emissions control and other areas of pollution abatement.
Much of the work has focused on developing a standard test for determining washcoat resistance to erosion. A Plexiglas apparatus was designed and built for testing 1” diameter x 1” long monoliths. Compressed air at 70 psi flows through a condenser to trap compressor oil, through a flow meter, and then through a distributor to ensure even flow through all of the monolith’s channels. Space velocities of up to 750,000 hr-1 are possible.
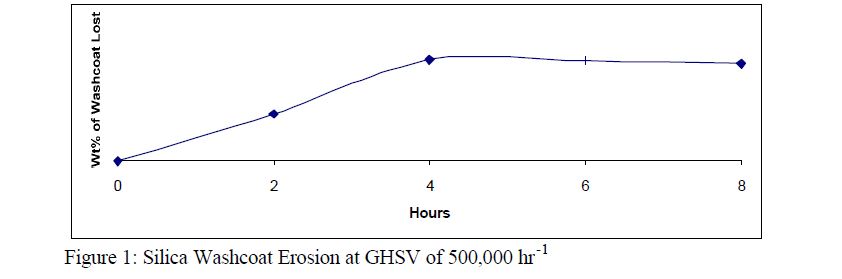
A standard erosion test is, putting the monolith in a dessicator overnight, weighing it immediately upon removal from the dessicator, putting it in the test apparatus, subjecting it to a space velocity of 500,000 hr-1 for 8 hours. The monolith is then removed from the apparatus and placed in the dessicator overnight and weighed the next day. The monolith has to be put in a dessicator because silica has a high affinity for water. If the monolith is not weighed after being in an environment with a constant absolute humidity there will be very little accuracy in measuring the weight of silica lost due to erosion, due to variable amounts of water absorbed on the silica. This standard erosion test was developed based on the typical data for monoliths with Davisil/Cab-O-Sil washcoat as seen in the Figure 1.
That only 3% of the washcoat is lost due to erosion, and the erosion ceases after only four hours demonstrates that a stable silica washcoat can be developed.
Preliminary experiments were performed to determine the most important procedural variables in preparing a monolith with a 10 wt% washcoat with the least number of slurry applications possible. 1” diameter x 1” long cordierite monoliths with 200 cells/in2 from Corning were used. The variables tested were: (1) the strength of the acid bath used to clean the monolith surface, (2) the slurry composition, (3) the drying temperature between slurry applications. These experiments showed that the strength of the acid wash affects the weight the monolith losses during the acid treatment but it does not change the number of applications needed to give the monolith a 10 wt% coating. Changing the composition of the slurry has a large effect on the effectiveness of the procedure. Increasing the wt% of Cab-o-sil M-5 in the slurry greatly reduces the number of applications needed, but slurries with greater than 8 wt% Cab-o-sil have viscosities large enough that the slurry does not easily flow through the monolith channels. Increasing the wt% of Davisil-644 in the slurry does not significantly increase the viscosity; it has not yet been determined if a high wt% of Davisil will reduce the number of applications. Only 4 applications were needed with a slurry containing 8 wt% Cab-o-sil/ 2 wt% Davisil, while up to 20 applications were needed for a slurry containing 2 wt% Cab-o-sil/ 8 wt% Davisil. It was observed that high drying temperatures (i.e. 500 oC) between applications caused the washcoat to crack and erode more readily. Drying at a temperature as low as 80 oC minimizes cracking but significantly increases the drying time.
Surface area tests show that the coating procedure does not change the surface areas of Cab-o-sil and Davisil. Samples of Cab-o-sil and Davisil were subjected to the same drying and calcining treatments used in preparing the monoliths and no loss or gain of surface area was seen.
The literature reveals that a successful way to make a stable washcoat is to make the washcoat of a composite material.1 Composite silica washcoats can be made using silicas with different particle size distributions. One type of particle should have a significantly larger average particle diameter than the other so the smaller particles can fill in the voids. Tests have been done to evaluate the grinding time needed in a ball mill to reach the desired particle sizes for Davisil- 644. Particle size distributions were determined using a Coulter-Counter LS100 Particle Analyzer. Solutions can be made that have particles with average particle sizes ranging from 1 ìm – 150 ìm.
The research completed thus far shows: (1) monolithic ceramics can be coated with high surface area silicas, and (2) these coatings are stable against erosion. The research also shows that slurry composition and the drying temperature between coats are preparation variables that need to be explored to optimize the coating process.
Tests will be conducted to develop a technique for coating the monolith after treating the monolith in a vacuum to remove gases from the pores, thus enabling the slurry to enter the pores more readily. A statistically designed factorial experiment will be performed to determine the effects of drying temperature, slurry composition, and type of application (vacuum or pouring) on the number of slurry applications needed to achieve a 10 wt% coat, washcoat erosion, and washcoat surface area. Two different slurry compositions will be used:
1. A slurry with a combination of large and small particles, about 20 ìm and 1 ìm respectively, of Davisil-644. This slurry will consist of about 50 wt% Davisil in water.
2. A Cab-o-sil/Davisil slurry with 5 wt% Cab-o-sil. The Cab-o-sil will give the solution adhesive properties and at this wt% will not be too viscous, to pour through the monolith channels. The Davisil will have an average particle size of 20 ìm and the wt% will depend on the amount of Davisil that can be added without making the slurry to viscous.
Reference
- Tsang, Chih-Hoa M., Bedford, Raymond E. 1992, US Patent 5,114,901.