Peter M. Crapo and Dr. Kenneth Solen, Chemical Engineering
Stroke, heart attack, or other severe medical problems may arise when platelet aggregates block a patient’s blood vessels. These aggregates form when blood is exposed to foreign surfaces in biomedical equipment. When blood is first exposed to a foreign surface, platelets adhere to the surface. A cluster of platelets on the surface is called a thrombus. Thrombi grow as more platelets adhere, and the momentum of passing fluid or other causes may dislodge them. Dislodged thrombi are called emboli, and the entire process is called thromboembolism. Thromboembolism cannot be entirely avoided in blood-contacting devices, such as artificial organs.
A graduate student in the Biomedical Engineering Laboratory at BYU is currently developing a computer model of thromboembolism (1). An accurate model could reduce risk to patients through better design and evaluation of biomedical equipment. Acceptance and use of this model will depend on its ability to correctly predict thrombus formation and probability of embolization under a wide range of conditions. There are many variables that must be understood to successfully construct a model.
Some preliminary data from the laboratory indicated two variables of major importance for the model; the level of heparin (an anticoagulant) added to the blood and the material choice. Different concentrations of heparin and different blood-contacting materials produce varying degrees of thromboembolism. Heparin is usually added to blood to prevent clotting, and it is commonly thought to reduce thromboembolism, too. Reducing thromboembolism is useful when blood is being passed through biomedical devices and returned to a patients body. Unfortunately, if too much heparin is injected into the patient then excessive bleeding occurs during surgery. An optimal level of heparin will reduce thromboembolism without endangering the patient. An optimal material will minimize thrombus formation in biomedical equipment. Only the effect of material was studied because of the time-intensive production of flow cells.
Four materials were tested: siloxane or silicone rubber (Si), polyethylene (PE), polyvinyl chloride (PVC), and Teflon FEP (Tef). Polymers are good biomaterial candidates because of their relative flexibility, low cost, high strength, and hemocompatibility. Fresh blood was drawn into four heparinized syringes (1.0 U/ml) from one of five donors by a certified phlebotomist and then passed through four parallel flow cells (0.75 ml/min), one of each material. All four flow cells were immersed in saline and placed under a microscope, providing twenty minutes of visual observation and data per experiment.
Each flow cell was constructed by snugly inserting tubing segments, each one millimeter long and one millimeter apart, into a single longer tubing segment with a larger inner diameter. Two tubing segments were originally planned for the flow cells, but greater embolization was observed using four segments as shown in Figure 1. A higher reaction was necessary to more clearly separate the blood response to different materials. The four shorter segments, called flow steps, provided conditions that caused thromboembolism. The longer segment was thirty centimeters long, allowing for the attachment of a light scattering microemboli detector (LSMD) downstream from the flow steps. The LSMD counted the number of passing emboli and collected data for each different material.
Instead of completing two experiments for each of three donors, the project was altered to four experiments for each of five donors. This minimized the possible impact of poor donor selection on the quality of results. Experiments were run immediately following venipuncture. All results shown were obtained between March 27th and May 25th, 2001.
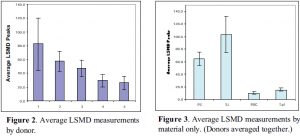
The results of this study shown in Figure 2 indicate two classes of donors: donors 1, 2, and 3 as opposed to donors 4 and 5. Adding other donors should confirm these distinctions or create statistically significant differences to distinguish some subsets. Figure 3 shows that Si was generally the most thrombogenic material tested, followed by PE. Statistical analysis showed that PVC and Tef were not significantly different, but their responses were low enough to require more data before being classified together.
A reliable computer model of thromboembolism will provide an invaluable tool to designers and evaluators of biomedical devices. This will greatly reduce the danger to clinical patients. The foremost purpose of this continuing research is the progress of medicine and its patients.
References
- Goodman PD, Hall MW, Sukavaneshvar S, Solen KA: In vitro model for studying the effects of hemodynamics on device induced thromboembolism in human blood. ASAIO J 46: M576-M578, 2000.