Timothy M. Allred and Dr. Dale R. Tree, Mechanical Engineering
The emissions produced by both passenger and commercial vehicles are a significant contributor to pollution problems that face many cities and areas around the world. One of these pollutants is known as NOx, which refers to the chemical compounds NO2 and NO. These gases are formed when fuel is burned at high temperatures and are an undesirable by-product of the internal combustion engine. Nitrogen dioxide reacts in air to form acid rain as well as contributing to ground-level ozone and smog.
In addition to NOx, diesel engines produce solid particulate matter, which has been linked to increased incidents of respiratory problems and increased rates of cancer. It is a concern of the diesel engine industry that changes in fuel composition intended to reduce particulate matter will also result in an increase in NOx emissions. Oxygenated fuels are of particular interest because the increased amount of oxygen reduces particulate formation but provides the potential for increased NOx formation through increased N2-O2 reactions.
Dr. Dale R. Tree and his PhD student William Cooley have tested one oxygenated fuel, diethyl ether, and shown that when compared on an equal temperature basis to diesel fuel, the amount of NOx emissions are not significantly different for the two fuels. My research objective was to test another fuel to add to the current data set of NOx measurements for oxygenated fuels.
Experimental Procedure and Set-Up
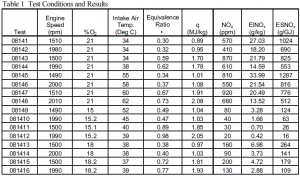
Testing was performed on a Cummins, 5.9 Liter direct injection diesel engine previously modified to a single cylinder engine for more accurate data acquisition. The proposed fuel, a mixture of tetraethoxypropane and heptamethylnonane, was abandoned because of chemical costs in favor of a new mixture of methoxyethyl ether and ethanol. Table 1 lists the test conditions as well as the results. The testing conditions varied were engine speed, equivalence ratio, intake oxygen concentration (%O2), and intake air temperature. These parameters were selected in order to achieve a wide variation in flame temperature and NOx formation rates. The following parameters were also recorded for each test: intake pressure, fuel flow rate, air flow rate, NOx in ppm, and exhaust pressure.
The initial fuel mixture of 25% methoxyethyl ether and 75% ethanol showed unfavorable engine running characteristics. Ignition of the fuel was delayed severely in the engine cycle. The mixture composition was changed to 50% of each chemical to improve engine running characteristics. A total of 24 test conditions were planned, yet only 16 were completed due to running out of test fuel. This should, however, be enough to create a sufficient data set.
Results and Discussion of Results
Ensemble averaged cylinder pressure data were collected for all of the tests but are not reported. Test conditions and tabular results are shown in Table 1. The results appear to produce reasonable trends and fall within the expected range. Trends in the NOx formed are seen to follow trends in expected flame temperatures with higher NOx being produced at conditions which produce higher flame temperatures. As engine speed increased, the amount of NOx decreased because of longer combustion duration and therefore a decrease in flame temperature. Also, as the intake air temperature was increased, flame temperature was increased and therefore NOx increased. Results are also as expected with a dramatic decrease in NOx when the intake oxygen concentration was reduced. This is due to the diluent effect of excess nitrogen which decreases the flame temperature. EINOx and ESNOx are other values of NOx normalized by the amount of fuel injected and by the lower heating value of the fuel respectively. These values follow the same trends as the NOx concentrations but are useful for comparing NOx obtained from engines burning different types of fuels. The values also follow expected trends.
Conclusions and Lessons Learned
This project was successful in collecting test data to add to the data set of NOx measurements for oxygenated fuels. This data will now be given to Dr. Tree for further analysis to determine if this oxygenated fuel does not significantly produce different NOx emissions when compared to diesel fuel on an equal temperature basis, and will eventually be used as part of a publication.
The most important lesson learned was that this type of experimental research does not involve just taking some data and analyzing it. It also deals with being able to solve problems encountered in malfunctioning equipment. This part of this research project took the majority of the time. Help from others including a lab assistant and faculty mentor was invaluable in learning how the equipment worked and how to troubleshoot the problems encountered. Learning a little bit about the process of troubleshooting problems will be invaluable to future research in graduate studies, my career, and also in daily life.