Nathan M. Cluff and Dr. Calvin Batholomew, Chemical Engineering
Our daily lives are dependent upon petroleum. This is evident in the fact that 26.4% of the United States’ energy consumption is used directly for transportation in the form of liquid fuels.1 To prevent shortages in gasoline, jet fuel, home heating oil, and other petroleum products, alternatives will be needed to supplement the rising world demand. As a citizen that will raise a family during a time when alternative energy sources will need to be implemented, I have a keen interest in how we will meet our energy needs and where we will get those resources. One alternative is the synthesis of these fuels from coal, known as Fischer-Tropsch Synthesis (FTS).
The process of synthesizing gasoline consists of first gasifying coal to form carbon monoxide, combining it with hydrogen to form long chain hydrocarbons, and then “cracking” the product into petroleum products. This process is a reality in South Africa and other locations where fullscale commercial application of FTS is currently in use. Even though the process is possible, economics are the major factor in preventing it from becoming wide spread. Under the high pressure and high temperature reaction conditions, the catalyst deactivates and must be replaced regularly. Replacement of the catalyst is a major expense associated with synthesis. Progress in the development of catalysts needs to be made to make petroleum synthesis economically feasible.
During the reaction process, water is formed as a by-product. Under the high-pressure and hightemperature conditions, it reacts with the catalyst’s silica support, resulting in mobile silicon hydroxyl groups. While the support, or silica, is in this condition, it agglomerates and renders the catalyst inoperative. A possible solution to agglomeration is to coat the support with a hydro-thermally stable compound. In the research presented here, a titanium perovskite was used as a thin film coating on the silica in an attempt to block the silica and steam reaction.
The titanate of choice is a perovskite of the chemical formula: Mg1Ti1O3. Two test samples were prepared. One sample was prepared with a 16 weight percent titanate, the other with 27 weight percent. These two weight percents were chosen based on calculations of how much titanate it would take to cover the entire surface of the silica powder with a coating that is a few atoms thick. The titanate coating was prepared by the impregnation of a silica support with magnesium nitrate and titanium ethoxide, in stoichiometric ratios. Following impregnation, the sample was dried at 80o C and calcined at 700o C to form the titanate-coated silica.
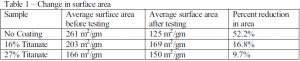
Silica normally has a high surface area, but when it begins to agglomerate, the surface area decreases. Therefore, measurements of surface area were used to determine if a thin coating would increase the stability of the silica. Davisil 654 was the type of silica used in this testing due to its larger pore size in comparison to other silica. Large pore size was useful because coating the silica had a tendency to plug smaller pores. The Davisil 654, before it was coated, had an average surface area of 261 m2/gm. Upon coating the silica, the surface area decreased, and after the lighter coating of 16% titanate, the surface area was 203 m2/gm. The heavier coating of 27% titanate resulted in a surface area of 166 m2/gm.
The support was tested by duplicating conditions that exist during typical FTS operations. Using sample sizes of 2-3 grams, multiple samples of coated and non-coated supports were steam treated at a pressure of 5 atm of water and at a temperature of 220o C. To ensure that the deterioration of the silica was measurable, each test ran for 72 hours. A duplicate of each test was performed to measure repeatability and ensure accuracy.
After the hydro-thermal stability testing was completed, the surface area of each sample was measured. The results, summarized in Table 1, are encouraging. While the coating did not completely stop the agglomeration of the silica, it did slow down its deterioration considerably. The heaviest coating provided the most protection to the silica. This was noted by a reduction in area of only 9.7% compared to a 52.2 % reduction in the sample with no coating.
The possible relevance of these results is that by applying this to future catalysts, it is expected that it can increase the life expectancy of the catalysts. This is valuable because using less catalyst may lower expenses for companies that employ Fischer-Tropsch Synthesis. Since these results are encouraging, there is a possibility that future investigation of the enhancing properties of perovskite coatings will be beneficial.
There are other aspects which need to be examined before this coating can be used with confidence. There is a possibility that the titanate may interact with the actual catalytic material resulting in a detrimental or beneficial effect. Actual synthesis testing is recommended to discover possible interactions. Also, variations in flow rates, temperatures, or pressures may influence the stability of the coating. Further hydro-thermal stability testing is recommended to gain a comprehensive understanding of the consequences of using this titanate coating.
References
- 1999 United States of America Annual Energy Review, pgs 8,117