Jeffery K. Thomson and Dr. Eric T. Sevy, Chemistry and Biochemistry
The kinetics of unimolecular reactions are especially interesting in the manner in which they absorb or transfer energy. For a unimolecular reaction to proceed, an activation energy barrier must be overcome. This barrier can be overcome through collisions with other molecules or atoms with sufficient force that excite vibrations violent enough to break chemical bonds. It has been observed that some molecules are more efficient at transferring energy through collisions than others. The reasons why some molecules transfer energy more efficiently are not fully understood. The goal of this project is to provide a window into the events taking place during and immediately after a molecular collision.
The energy a molecule gains from a collision is stored in one of three ways: translational, rotational or vibrational motion. If the energy a molecule acquires through a collision is distributed over several distinct vibrations or rotations in the molecule, none of which have sufficient energy to break bonds, the result will be an energized but unreacted molecule. If this energized molecule (donor) then collides with other ground state molecules (bath), the donor can transfer energy to the bath thereby being deactivated.1
If the donor molecule doesn’t immediately collide with a bath molecule after becoming energized, the molecule’s stored energy can redistribute itself over the molecular framework, thereby enhancing some vibrational modes often to the point where a reaction is possible. To study intramolecular vibrational redistribution and energy transfer efficiency, molecules with a single vibrational quantum state need to be prepared. The technique we proposed to use to accomplish this task is called stimulated Raman pumping spectroscopy.2 Acetylene gas was selected as the potential donor molecule, and we intended to prepare acetylene molecules in a symmetric stretch quantum state followed by an asymmetric stretch quantum state and determine which state transferred energy more efficiently. However, this process was more difficult than we anticipated. Our attempts thus far to accomplish this task have failed.
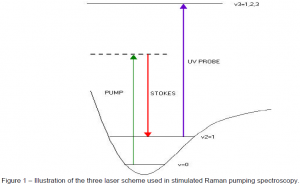
Stimulated Raman pumping spectroscopy has its roots in a similar technique called stimulated emission pumping spectroscopy (SEP).3 Both techniques involve a three laser scheme (Fig. 1). One laser beam, the pump beam, stimulates a molecule to an electronically excited state. Under normal conditions the excited electron cascades back down to the ground state and fluoresces in the process. If another laser beam, termed the Stokes beam, stimulates an emission from the electron before it has a chance to fluoresce, a molecule in a single vibrational state can be prepared. The third laser beam, termed the probe beam, can then be used to characterize this vibrational state. Stimulated Raman emission pumping offers some distinct advantages. This method relies on the scattering of incoming laser radiation by a molecule. Rather than being excited to a discrete electronic state, the molecule is excited to a virtual state, meaning it has no discrete energy level correspondence. The dump beam is then used to stimulate an emission from this virtual state to a single vibrational state. A probe laser is then used for characterization as in SEP. An advantage of the stimulated Raman pumping technique is that the wavelength of the pump beam can be somewhat arbitrary since it doesn’t have to correspond to a discrete electronic state transition. Another advantage is that molecules can only be in one of two states, the ground state or the prepared vibrational state whereas in SEP the molecules can exist in a number of different states.
Difficulties with this technique emerged as we were attempting to record a fluorescence spectrum of acetylene using our setup. This step is necessary in order for us to determine which wavelengths to use for the Stokes beam to excite the wanted vibrational quantum state. We couldn’t get enough power out of the laser to sufficiently excite the acetylene molecules to a level we could detect. We attempted to boost laser power by doing a laser realignment. We were able to boost the laser power by nearly twenty percent, but still found it to be insufficient. We are currently researching other methods by which we may proceed. It became apparent to me that to complete a project of this magnitude a time commitment on the order of a few years rather than a few months would be required.
References
- Sevy, E. T.; Rubin, S. M.; Lin, Z.; Flynn, G. W. Translational and rotational excitation of the CO2 (0010) vibrationless state in the collisional quenching of highly vibrationally excited 2- methylpyrazine: Kinetics and dynamics of large energy transfers. J. Chem. Phys. 2000, 113, 4912-4932.
- Chadwick, B.L.; Orr, B.J. Raman-ultraviolet double resonance in acetylene: Rovibrational state preparation and spectroscopy. J. Chem. Phys. 1992, 97, 3007-3020. 3Kittrell, C.; Abramson, E.; Kinsey, J.L.; McDonald, S.A.; Reisner, D. E.; Field, R. W. Selective vibrational excitation by stimulated emission pumping. J. Chem. Phys. 1981, 75, 2056- 2060.