David Simpson and Dr. John Lamb, Chemistry and Biochemistry
Awareness of the potential contamination issues with perchlorate ion (ClO4 -) came about in 1985 when the Environmental Protection Agency (EPA) first reported contamination in 14 Californian wells ranging from 0.11 to 2.6 micrograms per liter (ppm).1 In 1992 the EPA issued a provisional reference dose (RfD) of 0.14 milligrams per kilogram per day, which set a standard of 18 parts per billion (ppb) in drinking water.2 However, in January 1997 the EPA indicated that a reporting limit of at least 4 ppb would be necessary; yet, procedures to measure perchlorate ion at such low levels were unavailable.3 By March of that same year, advances of ion chromatographic (IC) methods had improved detection limits from 400 ppb to 4 ppb. Within several months of developing this technique, perchlorate was discovered at various manufacturing sites and in wells and drinking water supplies throughout the United States.
In 2002, a revised provisional RfD of 0.00003 mg/kg per day (drinking water standard of 1 ppb) was proposed after additional studies were performed on potential toxicity. However, procedures for detecting such low-level concentrations are again unavailable and are currently under development.
Perchlorate is both a naturally occurring and man-made chemical that is found in a variety of applications from solid rocket propellant to air bags to paints and enamels.4,5 As a result of such processes, perchlorate is being increasingly discovered in soil and water. Studies into perchlorate toxicity have shown that it interferes with iodide uptake into the thyroid gland, which disrupts thyroid functions associated with regulating metabolism.6
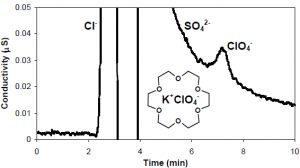
Our proposed objective was to utilize an organic modifier, 18-crown-6, in the mobile phase of an underivatized IC column to achieve retention and separation of trace-level perchlorate ion from common anions found in complex drinking water matrices. Recent research in our laboratory has shown that the addition of 18-crown-6 to the mobile phase of an underivatized-MPIC resin column produces the separation of both common inorganic and polarizable anions. Retention of the anions is a reflection of their ability to interact with the macrocyclic ligand.7 The advantage of using a mobile-phase functional site, such as 18-crown-6, as opposed to a stationary-phase site is that the retention capabilities of the column can be easily modified by simply varying the number 18-crown-6 complexes formed in the eluent. This great flexibility allows for the selective retention and placement of specific analyte ions. Perchlorate as a larger and polarizable anion readily interacts with the macrocyclic ligand, while common anions as smaller and tighter spheres are limited in their interactions with the ligand. Thus, by simply optimizing the concentrations of 18-crown-6 and potassium hydroxide in the eluent, we have been able to achieve a detection limit for perchlorate ion to less than 1 ppb in spiked Provo City water samples (Figure 1).
Having achieved a detection limit below the proposed standard, our findings were presented to the EPA as a new and alternative method for quantifying low-level perchlorate ion in water systems. Although we have shown its practicality in spiked Provo City water, the EPA felt a few more experiments would be necessary to prove that the proposed method could be applied to a whole spectrum of water matrices. The most rigorous of these tests is to separate and accurately quantify trace-level perchlorate in a laboratory-generated standard containing 1000 ppm of sulfate, chloride, and carbonate ions, which greatly exceeds the concentration of these common anions found in our water systems. If our proposed system is able to detect trace-level perchlorate under these conditions, then our method should be capable of separating and quantifying perchlorate ion to concentrations less than 1 ppb in most water system throughout the United States. If verified, these results may then help the EPA to establish a new RfD value for perchlorate and lower the toxicity risks associated with its consumption.
The current state of our research has focused on achieving detection of trace-level perchlorate ion under these high-salt conditions. We have already preliminary shown that separation and detection is possible under the suggested EPA experiments, but continued research is required to optimize the conditions of the ion chromatographic system necessary to consistently achieve levels below 1 ppb. In future work, we also hope to obtain water samples from across the nation to more accurately show the versatility of our proposed method to a broad range of water matrices.
References
- Takata, K. (1985) San Francisco, CA: U.S. E.P.A., Superfund Records Center, Region 9; December 23.
- Dollarhide, J. S. (1992) Cincinnati, OH:U.S. E.P.A., Environmental Criteria and Assessment Office; December 2.
- U.S. Code. (1996) Safe Drinking Water Act, as amended by PL 104-208, September 30. U. S. C. 42: § 300f et seq.
- Rogers, D. E., Lt. Col. (1998) Wright-Patterson Air Force Base, OH: Department of the Air Force, Air Force Materiel Command Law Office; October 30.
- Siddiqui, M.; LeChevallier, M. W.; Ban, J.; Phillips, T.; Pivinski, J. (1998) Vorhees, NJ: American Water Works Service Company, Inc.; September 30.
- Argus Research Laboratories, Inc. (2001) Horsham, PA: Protocol no. ARGUS 1416-003.
- Lamb, D. J.; Smith, R. G. J. Chromatogr. 1993, 640, 33–40.