Aaron Secrest and Dr. Craig Thulin, Chemistry and Biochemistry
Age-related macular degeneration (AMD) is the leading cause of irreversible visual impairment in the elderly and is currently untreatable. In the central region of the posterior retina (the macula), AMD is caused by damage to and subsequent death of retinal pigment epithelial (RPE) cells and, consequently, the photoreceptors that are attached to them, leading to vision loss. The current thinking in the field of ophthalmology is that the eye cannot function efficiently after 50-60+ years of continuously repairing damage caused by exposure to high-energy photons from blue light. The increase in average human life span correlates with a higher prevalence of AMD.
The autofluorescent age pigment known as lipofuscin is a complex lipid-protein aggregate that accumulates with age in highly metabolic differentiated cells, including human retinal pigment epithelial cells.1 Granules of lipofuscin are known to be very photoreactive to blue (visible) light, producing an array of reactive oxygen species (ROS).2 ROS can cause two scenarios in human cells: (1) irreversible DNA damage, leading to programmed cell death (apoptosis), or (2) mutations in crucial DNA sequences that could lead to tumorigenesis (cancer). Lipofuscin has been reported to occupy up to 19% of cytoplasmic volume in the retinal pigment epithelial cells at age 80.3 Recent studies show that the cell viability of lipofuscin-fed RPE cells exposed to blue light decreased substantially within 24 hours in vitro.4 The same report noted key morphological changes in the cellular membrane, namely, membrane blebbing, which is a distinguishing physical characteristic of apoptosis. Although apoptosis can be inferred, it has not been confirmed experimentally, and the molecular mechanism by which this cell death occurs is currently unknown.
However, A2E, a major fluorophore in lipofuscin, has been shown to induce apoptosis when exposed to blue light. The pathway through which this apoptosis occurs is a caspase cascade regulated by the Bcl-2 protein located on the mitochondrial membrane.5 Caspases are proteases that systematically degrade essential cellular proteins, which are then packaged into small vesicles to be taken in by neighboring cells and macrophages, preventing inflammation of the tissue; hence, apoptosis is referred to as programmed cell death.
Results and Discussion
In our first experiment to directly compare the light-induced cell death of lipofuscin-fed and A2E-fed human RPE cells, our main focus was to maintain the cells in a humidified incubator at constant 37°C. We powered a 50 W halogen bulb using a 12 V battery, and we estimate that the cells were exposed to white light with an intensity of ~5 mW/cm2 for 1.3 h. The results of this experiment are shown in Figure 1A.
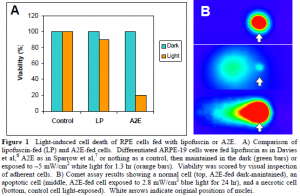
For our second attempt, we focused on providing a bluelight exposure of 2.8 mW/cm2, while maintaining the cells at a constant temperature of 37°C. We again utilized a 50 W halogen bulb situated 6 inches above the cells. A petri dish filled with water absorbed both infrared and heat, and a piece of cobalt glass filtered the visible light, allowing only blue light (390-550 nm) at an intensity of ~3 mW/cm2 to reach the cells. For currently unknown reasons, our negative control cells died when exposed to this intensity of light. We performed a neutral comet assay on the cells, and examples of the various types of cell death are shown in Figure 1B.
Conclusions
• Both A2E-fed and lipofuscin-fed human RPE cells undergo cell death to some extent (80% and 10% decrease in viability, respectively) when exposed to white light at an intensity of ~5 mW/cm2 for 1.3 h.
• When exposed to blue light at an intensity of approximately 3 mW/cm2 for 24 h, both A2Efed and lipofuscin-fed human RPE cells undergo cell death characteristic of apoptosis. However, negative control cells also significantly decreased in cell viability under the same conditions.
References
- R. W. Young. Surv Ophthalmol. 32, 252-69 (1988).
- W. T. Ham, J. J. Ruffolo, H. A. Mueller, D. Guerry. Vision Res. 20, 1105-11 (1980).
- L. Feeney-Burns, E. S. Hilderbrand, S. Eldridge. Invest Ophthalmol Vis Sci. 25, 195-200 (1984).
- B. S. Winkler, M. E. Boulton, J. D. Gottsch, P. Sternberg. Mol Vis. 5, 32-42 (1999).
- J. R. Sparrow, B. Cai. Invest Ophthalmol Vis Sci. 42, 1356-62 (2001).
- S. Davies, M. H. Elliott, E. Floor, T. G. Truscott, M. Zareba, T. Sarna, F. A. Shamsi, M. E. Boulton. Free Radic Biol Med. 31, 256-65 (2001).
- J. R. Sparrow, C. A. Parish, M. Hashimoto, K. Nakanishi. Invest Ophthalmol Vis Sci. 40, 2988-95 (1999).
