Eric Merkley and Dr. Earl M. Woolley, Chemistry and Biochemistry
Apparent molar heat capacity, Cp,f, and apparent molar volume, Vf, are valuable thermodynamic quantities in the study of solute-solvent interactions and chemical reactions in aqueous solutions. Work in our laboratory1-3 has demonstrated that fixed-cell differential scanning calorimetry (DSC) and vibrating-tube densimetry can provide sensitive and accurate data for calculating Cp,f and Vf. We have recently collected data for solutions of copper(II) nitrate, nickel(II) nitrate, and zinc(II) nitrate. As for most compounds, there is a lack of Cp,f and Vf date for these heavy metal nitrates. Our measurements will allow calculation of Cp,f and Vf.
We purchased these reagents as hydrated salts with the formula M(NO3)2 · nH2O, where n is the degree of hydration and M is Zn, Cu, or Ni. The value of n influences the calculated concentration of a solution made by mass. Accurate, precise concentration is essential in calculating apparent molar volume and apparent molar heat capacity. Preliminary calculations of Cp,f and Vf for copper(II), zinc(II), and nickel(II) nitrates show good agreement with literature data, if we make assumptions about the degree of hydration. We here report analysis of our Zn(NO3)2 and Ni(NO3)2 solutions by complexometric titration with ethylenediamine tetraacetic acid (EDTA), and analysis of our Cu(NO3)2 solutions by the iodometric method.4
Materials and Methods
We purchased standard EDTA (0.0101 M) from Aldrich, crystalline Zn(NO3)2 and Ni(NO3)2, (both 99.999% pure, metals basis) from GFS Chemicals, and Cu(NO3)2 from Aldrich (99.99% pure, metals basis). All other chemicals were reagent grade. We performed titrations with a buret and stepper motor controlled by a National Instruments LabView program. We calibrated the buret by using the mass of water per buret step, and the known density of water.
We carried out the EDTA titrations essentially according to the procedure of Swift and Butler.4 Standard EDTA solution was titrated into a solution of the analyte buffered with 1.2 F ammonium chloride at pH 10. We used the color change of a metallochromic indicator [murexide for nickel(II) and Eriochrome Black T for zinc(II)] to detect the endpoint.
We performed copper analyses by the iodometric method according to the procedure of Swift and Butler.4 Copper solution samples were heated to fuming in sulfuric acid, the pH was adjusted, an excess of potassium iodide was added, and the resulting tri-iodide ion was titrated with sodium thiosulfate. A standard solution of copper was prepared by dissolving a known mass of reagent grade copper foil in nitric acid and diluting with water. This solution was used to standardize a ~0.01 M solution of sodium thiosulfate
Results and Discussion
We conducted these analyses either on actual solutions of copper(II) nitrate, nickel(II) nitrate, and zinc(II) nitrate that were used in our densimetric and calorimetric experiments, or on dilutions of these solutions. We analyzed one solution of each compound, except for nickel(II) nitrate, in which case 2 solutions were analyzed. To calculate the extent of hydration n, we used our titration results and a Microsoft Excel spreadsheet containing the masses of reagent and water used to make all solutions, and Microsoft Excel’s Solver feature. This spreadsheet not only gives the value of n, but also the concentrations of all the solutions used in the calorimetric and densimetric experiments.
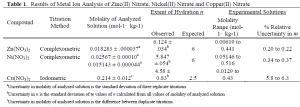
The results of our titrations are listed in Table 1, along with values of n, and the molality range of the actual solutions used for calorimetric and densimetric experiments. The results for zinc(II) and nickel(II) nitrates are precise and will allow the calculation of Cp,f and Vf for these compounds. The iodometric copper determination results are insufficiently precise for our purposes and are considered preliminary. We will use the results for the zinc(II) and nickel(II) nitrates to write a manuscript for publication of our recalculated Cp,f and Vf results.
References
- Ford, T.D.; Call, T.G.; Stark, M.A.; Origlia, M.L.; Ballerat-Busseroles, K.; Woolley, E.M. J. Mol. Liq. 2001, 90, 140–148.
- Patterson, B.A.; Woolley, E.M. J. Chem. Thermodyn. 2002, 34, 535–556.
- Call, T.G.; Ballerat-Busseroles, K.; Origlia, M.L.; Ford, T.D.; Woolley, E.M. J. Chem. Thermodyn. 2000, 32, 1525–1538.
- Swift, E.H.; Butler, E.A. Quantitative Measurements and Chemical Equilibria; W.H. Freeman and Sons: San Francisco, 1972; 457–488, 604–615.