Clinton R. King and Dr. Gary D. Watt, Chemistry and Biochemistry
We are investigating the kinetic effect of salt concentration on the reaction N2 + (6+2n)H+ + (6+2n)e- + 2(6+2n)ATP 2NH3 + nH2 + 2(6+2n)Pi + 2(6+2n)ADP (1) catalyzed by nitrogenase where the spontaneous decomposition of S2O4 2- is the electron source. Under high partial pressures of N2, n has a limiting value of one, but in the absence of N2, nitrogenase simply catalyzes the reduction of protons to H2.
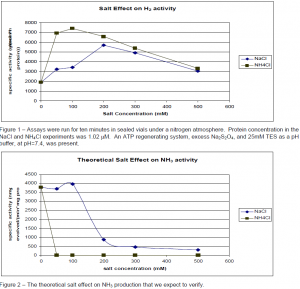
We observed that the concentrations of various salts (including buffers) affect the activity of the nitrogenase system differently (Figure 1). The experiments that produced figure 1 were performed under a nitrogen atmosphere. The difference in H2 production in the presence of NH4Cl and NaCl suggest that the two salts interact differently with the protein. It also suggests that the buffering system may make a difference. Indeed, that difference has been observed. A protein with an activity of 1903 nmol H2 evolved/(min*mg protein) with 25mM TES (Ntris( hydroxymethyl)methyl-2-aminoethanesulfonic acid) buffer was observed to have an activity of 2410 with 30mM phosphate buffer.
In order to confirm these effects it is desirable to measure the NH3 production under these conditions. Historical methods of measuring NH3 (NH4 + at this pH) have involved microdistillation of the ammonia and a color producing reaction. These have proved unreliable. We attempted to implement a method described by Dilworth et al,1 but this too proved unsuccessful after many trials with standards. The measurement of small concentrations of ammonium in the presence of large concentrations of sodium is a problem addressed by manufacturers of ion chromatographic systems. Richens et al2 recently published a method of quantifying ammonium in a large background by ion exchange chromatography. This research was performed in Dr. Lamb’s laboratory in our own department. They have agreed to collaborate with us and measure the ammonium in our samples. Figure 2 shows our expected results.
An examination of Figure 2 shows that we wish to measure, in 1mL assays, mM changes of NH4 + in the presence 0–.5 M NH4Cl. We propose to accomplish this with the use of 15N2 as the nitrogen source for (1). Because naturally occurring nitrogen is over 99% 14N, we will be able to distinguish between the 14NH4 + initially present and any 15NH4 + that the reaction might produce. Upon quenching, we will make the solution basic and release NH3 into the sealed atmosphere. We will withdraw some gas from the headspace and inject it into a mass spectrometer, which has the sensitivity to allow us to determine the amount of 15NH3 produced by nitrogenase in the presence of NH4Cl.
Based on the Thorneley-Lowe3 kinetic theory of nitrogenase, the slow step of (1) is the dissociation of the two nitrogenase proteins. We believe that the general salt effect accelerates this step by decreasing the energy of dissociation. We believe that the specific effect of NH4 +/NH3 is to associate with the active site of nitrogenase and prevent N2 reduction.4
References
- Dilworth, M. J.; Eldridge, M. E.; and Eady, R. R. Correction for Creatine Interference with the Direct Indophenol Measurement of NH3 in Steady-State Nitrogenase Assays. Anal. Biochem. 1992, 207, 6–10.
- Richens, D. A.; Simpson, D.; Peterson, S.; McGinn, A.; and Lamb, J. D. Use of mobile phase 18-crown-6 to improve peak resolution between mono- and divalent metal and amine cations in ion chromatography. J Chrom. A. 2003, 1016, 155–164.
- Lowe, D. J.; and Thorneley, R. N. F. The Mechanism of Klebsiella pneumoniae Nitrogenase Action. Biochem. J. 1984, 224, 877–907.
- The author wishes to acknowledge interpretive help of Phil E. Wilson, PhD candidate.