Jason Kenealey and Dr. Gerald Watt, Chemistry and Biochemistry
Ferritin is a protein that facilitates iron metabolism in the body. Virtually all living organisms are dependent on ferritin for iron storage. It has been found in everything including animals, plants and bacteria. Ferritin is formed from 24 subunits, which are arranged to form a sphere with a hollow center. In mammals these subunits consist of two isomers: heavy and light, which are named so due to the difference in their molecular weight. In the center the iron is stored as ferric hydroxide. The mechanism of the iron deposition into the core is not well understood. A part of this mechanism involves the binding of the ferrous ion to the protein shell.
Current research shows that the site where oxidation occurs is in the ferroxidase center, located in the heavy subunits. The purpose of this research is to determine the number of redox sites, which are thought to be where iron binds. This leads to the assumption that the redox centers are located on the heavy subunit to enable transport to the ferroxidase center. Animals are equipped with different ratios of heavy to light subunits. In order to determine if the number of redox sites are proportional to the number of heavy subunits, varying ratios of heavy to light subunits were used. This study used three different mammalian ferritins, namely; horse, sheep and rat ferritin. These ferritins have heavy to light ratios of 4 heavy to 20 light, 12 to 12, and 8 to 16, respectively.
This research will help to better understand the reaction mechanism for iron loading in the core of ferritin, by measuring the number of redox sites and determining if an increased number of redox sites speeds the rate at which iron is bound to the ferritin. To determine the number of redox sites, iron (II) was mixed with ferritin, at a 30 iron per ferritin ratio, allowing the iron to bind to ferritin in the redox sites. This was done aerobically so the iron would not load into the core. To measure the amount of ferrous that was not bound to the ferritin ortho-phenanthroline (o-phen), which chelated any ferrous that was unbound to the ferritin, was added in excess to the vial. As o-phen is added it chelated the ferrous ions that are unbound because o-phen has a higher binding constant with ferrous than ferritin. The rate of the ferrous being released by ferritin was measured by spectrophotometrically measuring the formation of the ferrous o-phen complex. To confirm these results I planned to use centrifuge tubes that had a membrane in them with pours which only allow unbound iron to pass. The unbound iron was then chelated with o-phen and the amount of iron was measure with a spectrophotometer. However, the preliminary experiments showed that to get any quantifiable data would be impossible. The membranes would bind the iron, and the amount of iron bound to the membrane varied so greatly that the percent relative error was too large to be able to establish a standard.
The results differed from the original hypothesis. In my hypothesis I expected that eventually all the iron would be released from the ferritin because the o-phen was in excess and has a higher binding constant. However, this was not the case. The o-phen removed the iron from the ferritin for a little while, but the reaction stopped before all the iron was complexed with the o-phen. The addition of dithionite (S2O4), a strong reducing agent, caused all the iron to be released by the ferritin, showing that the iron had been oxidized. There are three possible sources of oxidation the redox sites, oxygen, and the buffer. The buffer was eliminated by repeating the experiments in different buffers including water and observing no changes in the results.
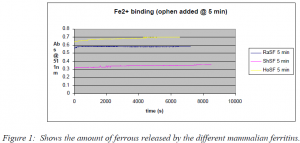
Although, painstaking effort was made to eliminate all oxygen from the solution there may have been a little remaining oxygen in the solution. This seems like it is a likely conclusion, because the amount of oxidized iron in the ferritin varied with each experiment, which correlates with varying amounts of oxygen contamination. However, besides this variable portion there is a qualitative difference between the amount of iron that was released by the different types of ferritin. The increased number of oxidized redox sites would allow for increased amount of iron loaded into the core of the ferritin. Figure 1 illustrates that the ferritins with the higher number of heavy subunits had more ferrous bound and released less upon the addition of o-phen. The increase in absorbance in the inividual samples is due to the iron being released from the ferritin. These irons are most likely bound to redox sites that are in the reduced form so the iron is not oxidized and is easily released. This leads to the conclusion that the number of iron binding sites is directly proportional to the number of heavy subunits. Also, as the number of heavy subunits increased the higher percent of the ferrous was oxidized and loaded into the core. Therefore, the heavy subunits must also contain a source of oxidation, which would be the oxidized redox sites.
The rate at which the iron is bound to the ferritin is difficult to measure due to this outside source of oxidation. This was the most difficult task to perform. I used many different techniques in order to remove all the oxygen from the solution so I could get results that I could compare in order to get a good rate for the binding. I continue to work on a solution for this problem. The problem is that there is such a low concentration of iron that it doesn’t take much oxygen contamination to alter the experiment enough to give results that are too variable to draw good conclusions from. If the problem can be solved adding additional iron, but there are several possible implications to additional iron. The introduction of a high amount of excess iron may affect the binding to the ferritin. If I try to keep the 30/1 ratio the same, but at higher concentrations, more ferritin is required which is expensive. There will also be additional error added because the solution will need to be diluted in order to get the absorbance of the solution low enough to get in the range of the spectrophotometer. These ideas will be applied as the research continues.