Scott Henneman and Dr. Steven A. Fleming, Chemistry and Biochemistry
Recent photochemical research has proven helpful in understanding the regio- and stereoselective mechanism of four-member ring formation. When compounds cycloadd— combine either inter- or intramolecularly to form rings—a broad assortment of isomers are generally formed. However, the regio- and stereoselectivity of the cycloaddition process—the tendency of the compounds to combine in specific ways, i.e. the preference for one product over another—can be greatly enhanced through the utilization of a tether group. This tether group can be anything used to control the reacting molecular components involved in a cycloaddition towards combination in a specific orientation. The regio- and stereoselectivity of the reaction, simply termed “selectivity” in general, is of particular interest in natural product synthesis to develop novel, efficient means of producing natural compounds or biologically active agents such as pharmaceuticals.
For a photoreaction to proceed, one of the reactants generally must enter a radical configuration wherein one of its electrons is in the ground state and another is in an excited state. When these two electrons have different spins, the reactant is said to be in the singlet excited state (S1), and when the electrons have the same spin, the reactant is in the triplet excited state (T1). It has been found that enones generally add to alkenes through the T1, and that arenes groups will add to alkenes via the S1 pathway. One compound, called 4-hydroxycoumarin, has both an enone and an arene group in its structure. Therefore, the use of 4-hydroxycoumarin tethered to an alkene group can elucidate the preference of the addition of the alkene to arenes and enones based on the excited state through which the molecule proceeds. Based on these photoaddition preferences of enones and arenes, two types of experiments were run. One with a triplet quencher, in theory facilitating the alkene addition through the S1, and the other type of experiment with a triplet promoter, allowing the reaction to more easily proceed through the T1. The investigation was an attempt to obtain information both about the regio- and stereoselectivity of the addition of a tethered alkene to 4-hydroxycoumarin, and about the preference of the alkene in adding to arenes and enones based on the promoted excited state.
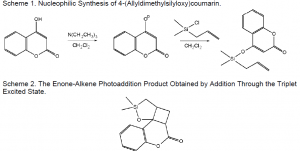
In order to tether 4-hydroxycoumarin to an alkene, a synthesis was devised. The resulting product, 4-(allydimethylsilyloxy)coumarin, was created using a straightforward nucleophilic substitution of allychlorodimethylsilane with the enolate of 4-hydroxycoumarin, formed by reaction with triethylamine (Scheme 1). Purification of the product through silica gel chromatography proved impossible because contact with the silicon caused degradation of the product via the formation of polymers. The formation of polymer, a solid white powdery substance, was verified via 1H NMR analysis. The monomer, the desired product, was a yellowish oil. This was obtained in 67% yield.
When 4-(allyldimethylsilyloxy)coumarin is irradiated, the carbon-carbon double bond on the enone absorbs light and is excited, presumably to a diradical. Acetone has a high triplet energy (~78 kcal/mole), and can thus act as a donor to increase the population of coumarin molecules in the triplet excited state. The increase in T1 population facilitated the intramolecular addition of the excited enone to the alkene in this experiment. This irradiation was performed twice—once for one hour, and once for two hours. The percent mass recovered from both the one- and two-hour reactions were 99% and 97%, respectively.
TLC evidence indicated the crude product contained several different photoproducts, and thus the product was purified by chromatography. Post-chromatographic yield was 89%. Multiplets at 4.9 and 5.8 ppm indicated that the allyl component remained in solution. However, the expected photoproduct, shown as 1 in Scheme 2, was also abundantly present. Exact yields could not be calculated, as similar polarities prevented 1 from being completely separated from the product containing the allyl component. Presence of 1 was verified by 13C NMR spectroscopy, COSY NMR, and mass spectrometry. The exact calculated mass of 1 is 260.0869. High-resolution EI mass spectrometric analysis found the observed m/z ratio of the peak at 260 as exactly 260.0869, with an error of –0.1 ppm and +0.0 mmu.
To facilitate the addition of the arene group on 4-hydroxycoumarin to the alkene, three different triplet quenchers were integrated into the reaction: 1,3-pentadiene, biphenyl, and p-terphenyl, with triplet energies of 59, 66, and 58 kcal/mol respectively. It was expected that the alkene would add to the aromatic ring on the coumarin, a reaction that would destroy the aromaticity of the compound and be easily detectable via 1H NMR. However, even after separating the crude material through chromatography, aromatic hydrogen peaks remained in every product. Thus, the triplet quenchers are presumed to have worked in the sense that they prevented the formation of 1, but did not facilitate the addition of the alkene to the aromatic group.